Old Ideas May Help Us Fight New Superbugs
33:33 minutes
Last week, the U.S. Army announced the arrival of a new enemy on American soil. But the danger was microscopic: a bacterial gene, easily passed to other bacteria, which confers resistance to one of our last-ditch antibiotics, colistin. The news was a reminder of our dwindling options in fighting superbugs. But researchers like Brad Spellman and Paul Turner are working on a few out-of-the-box ideas for the post-antibiotic era. Felicia Wu joins too, to talk about the lifecycle of an antibiotic, from the farm to your plate.
Read below to learn more about antibiotic resistance.

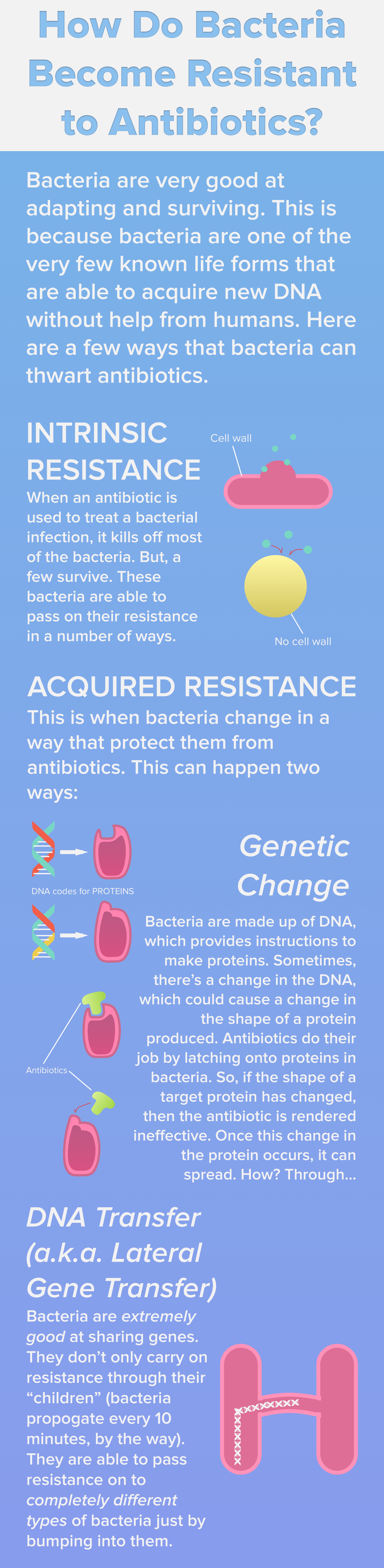




Paul Turner is a professor and the chair of the Department of Ecology and Evolutionary Biology at Yale University in New Haven, Connecticut.
Brad Spellberg is Chief Medical Officer at the LAC+USC Medical Center in Los Angeles, California.
Felicia Wu is John A. Hannah Distinguished Professor in the Department of Food Science and Human Nutrition at Michigan State University in East Lansing, Michigan.
IRA FLATOW: Last week, the US Army announced the arrival of a new enemy on American soil, but the danger was microscopic. Actually in the form of a bacterial gene, which confers resistance to one of our last ditch antibiotics, Colistin, and it can be easily transferred to other bacteria.
Don’t panic just yet, because the bacteria containing the gene weren’t resistant to our entire arsenal of antibiotics. So the woman who harbored the bacteria recovered and we’re not out of options just quite yet. But the news was a reminder that we are not winning many battles against antibacterial resistance, and we certainly are not winning the war.
There are, though, a few out in the box ideas that could help us get there. And my next guest is investigating one of them himself. Brad Spellberg is a Chief Medical Officer at LAC+USC Medical Center in Los Angeles. He joins us by Skype. Welcome back to Science Friday.
BRAD SPELLBERG: Thank you so much for having me.
IRA FLATOW: Brad, you were alarmed, were you, by the news of this Colistin resistant gene?
BRAD SPELLBERG: Well I wasn’t probably as alarmed as some of the media reports would suggest. It’s actually not new to have Colistin resistance. We’ve had Colistin resistance for a long time. The thing that’s new is that the resistance is now in a very transmissible form that can spread much more easily than prior forms of resistance. And if it does spread to bacteria that are resistant to everything except Callisto, we’ll start to develop widespread pan-resistant organisms that can’t be treated. That is alarming.
IRA FLATOW: Why are we running out of antibiotics?
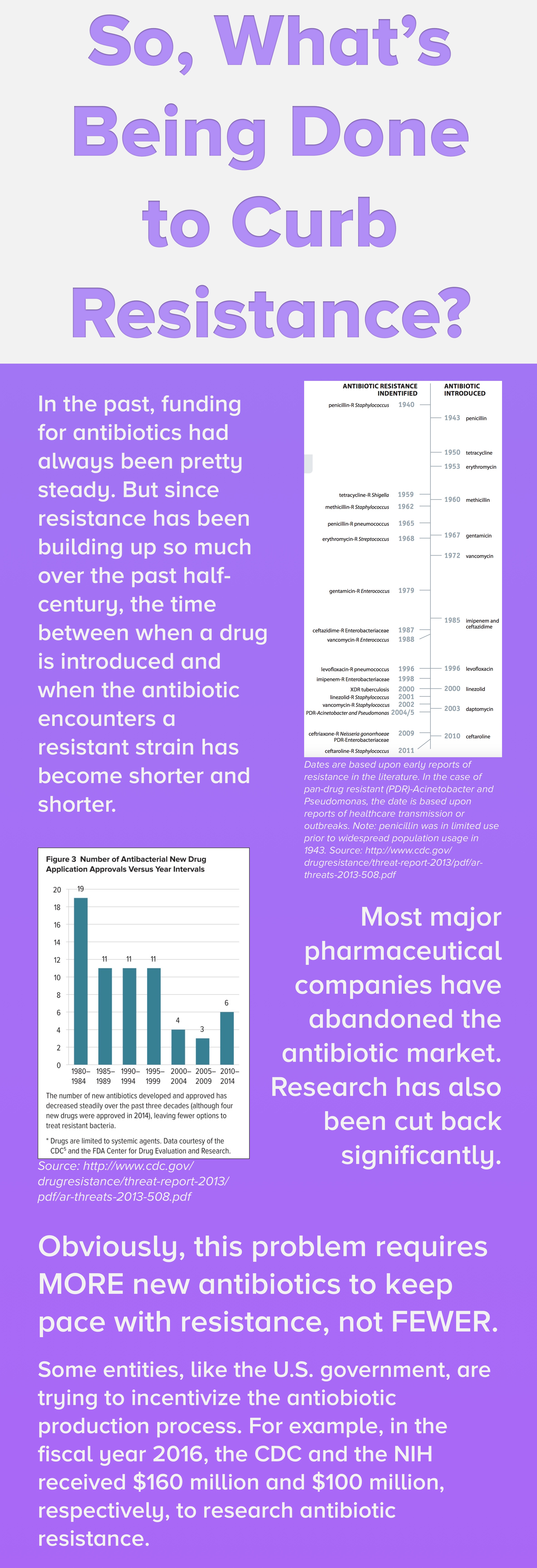
BRAD SPELLBERG: Well we aren’t getting new ones developed at nearly the rate they used to be. Because the science is increasingly difficult, the economics is not favorable for a variety of reasons to do antibiotic discovery, and the regulatory environment has been very hostile to new antibiotic development. On top of that, resistance continues to spread because we continue to so badly abuse antibiotics and overuse them.
IRA FLATOW: All right. The first part, if I understand in simpler terms, means that there’s not a lot of money to be made by drug companies to make antibiotics.
BRAD SPELLBERG: That’s certainly part of it. It’s not the only part of it. I mean, the science is actually really hard, because the stuff that’s easy to discover has already been discovered. So it’s getting increasingly difficult scientifically to discover the next new thing. And the regulations have really been very difficult to work with.
IRA FLATOW: You’ve said that one of the problems is not just running out of the drugs, but the way they’re administered. Like the oral form doesn’t work anymore versus the IV, which still works.
BRAD SPELLBERG: Well we have organisms that are typically encountered in health care settings that could be resistant to pretty much everything. But what you’re discussing is this much less well appreciated looming problem that I think has not gotten enough attention.
Which is that we’re now seeing community bacteria, not in health care settings, causing community onset infections– urinary tract infections, kidney infections, prostate infections, abdominal infections, like diverticulitis and appendicitis. They’re not resistant to everything. We have IV antibiotics left for them. But they’re resistant to everything oral.
Now if you’ve got a urinary tract infection, do you want me to have to put you in the hospital for a week on IV antibiotics? Of course not. You want your oral Cipro and go home. But we’re losing oral antibiotics for these patients. That’s getting very alarming.
IRA FLATOW: And the answer to that might be?
BRAD SPELLBERG: Well we need to do a much better job of protecting the antibiotics we currently have. We need to figure out how to get new antibiotics developed. And we need to think of new ways to treat infections that don’t try to kill the bacteria. Resistance occurs because we apply selective pressure by trying to kill the bacteria, so they emerge resistance. If we could treat infections without killing the bacteria directly, you might have a way to treat infections that’s less prone to inducing resistance.
IRA FLATOW: So what is the incentive for all of this new research that we need?
BRAD SPELLBERG: Well, that’s been very widely debated for a long time. For typical antibiotics, I think we’re going to be moving more and more over time towards a defense contractor model. Away from a traditional pharma model, where the government– and there are agencies that are capable and funded to do this, and they’re actually doing a very good job right now– can defray some of the upfront costs and risks to discover a new and develop a new antibiotic. On the back end, they get a say in which ones get developed, so we don’t get a bunch of [INAUDIBLE] drugs developed, but get drugs that we need to get developed and have a say in how the drugs will be priced downstream.
IRA FLATOW: But doesn’t the NIH do basic research?
BRAD SPELLBERG: NIH funds basic research. NIH also– as part of the Public Health Emergency Medical Countermeasures Enterprise, or PHEMCE– has established translational arms, where they can help both academics and small companies do manufacturing in toxicity testing and Phase I clinical trials to get drugs moved out of the laboratory and into patients, where they can then get passed on to an agency called Barda, which is in HHS, that can fund Phase II and Phase III clinical trials to get those drugs into patients.
IRA FLATOW: So what is it about– you mentioned the regulations that are tying up everything. What kinds of regulations?
BRAD SPELLBERG: The FDA has been very challenging in the antibiotics space for quite some time now. And they have made the conduct of clinical trials for new antibiotics very difficult, much larger than they used to be and much more difficult to get patients enrolled in the trials. And that causes the trials to be more expensive, they take longer to enroll, and there are people that just look at it and say, “Well I can’t do that trial. I just don’t think I can develop the drug.”
IRA FLATOW: I think we need to go back to loosening up some of that stuff.
BRAD SPELLBERG: Well I don’t like to use the word loosening because people think, oh you’re just trying to soften regulations. And I think the point is we definitely need regulations. Pharmaceutical industry is demonstrated without regulations there are horrible abuses. But the regulations have to make sense. And so what you want is trials that are feasible to enroll in the United States of America.
If you enroll a trial in Slovenia, which is where antibiotic trials are now enrolling– in Eastern Europe– how do I know that has any relevance to taking care of patients in the United States?
IRA FLATOW: Now you and others are researching something called antibody therapy, which actually hearkens back to an old treatment for infection, right?
BRAD SPELLBERG: Yeah it’s ironic. 120, 130 years ago, some of the original Nobel laureates in microbiology, after they discovered that germs cause infections, began learning that the blood produces weapons to defeat those infections. And so they started to immunize horses with various types of infections. And they would bleed the horse, take the serum out of the horse blood, and they would infuse that serum into patients. It was called serum therapy.
It was actually quite effective for diseases like diphtheria and pneumonia. It was also cumbersome. It had toxicities, because you’re using horse blood in humans. And it was so much more difficult, and somewhat less effective. When antibiotics came along, people just forgot about it and we abandoned it in the 1930’s and ’40s.
We’re now going back and learning that the primary thing in the blood that was working were these proteins called antibodies. And we have modern manufacturing methods. We can get very high purity, very high amounts of very specific antibodies to defeat microbes.
IRA FLATOW: Wow. And how effective– have we tried it yet? Is it working?
BRAD SPELLBERG: They are just now starting in the antibiotic space to get into clinical trials. What the irony is that because in fields like cancer and arthritis, there were not drugs as effective as antibiotics. Those investigators went to antibodies 20 years ago.
We’ve had antibodies that are incredibly effective for cancer and arthritis and inflammatory bowel disease for 20 years. Because antibiotics have been so effective, we’ve only more recently gone back to our roots in the infectious disease world. The lead antibodies are just now entering clinical trials. And I think that the first ones to hit the market will probably be there in maybe five years or so.
IRA FLATOW: I’d like to bring on another guest who’s using viruses that attack bacteria called bacterial phages, or phages for short, to make resistant bugs sensitive to antibiotics again. And details appeared in the journal scientific reports last week. Paul Turner, Professor and Chair of the Department of Ecology and Evolutionary Biology at Yale in New Haven. Welcome to Science Friday.
PAUL TURNER: Wonderful to be here. Thanks for having me.
IRA FLATOW: Tell us how this works. How do you make a resistant bugs sensitive to the antibiotics again?
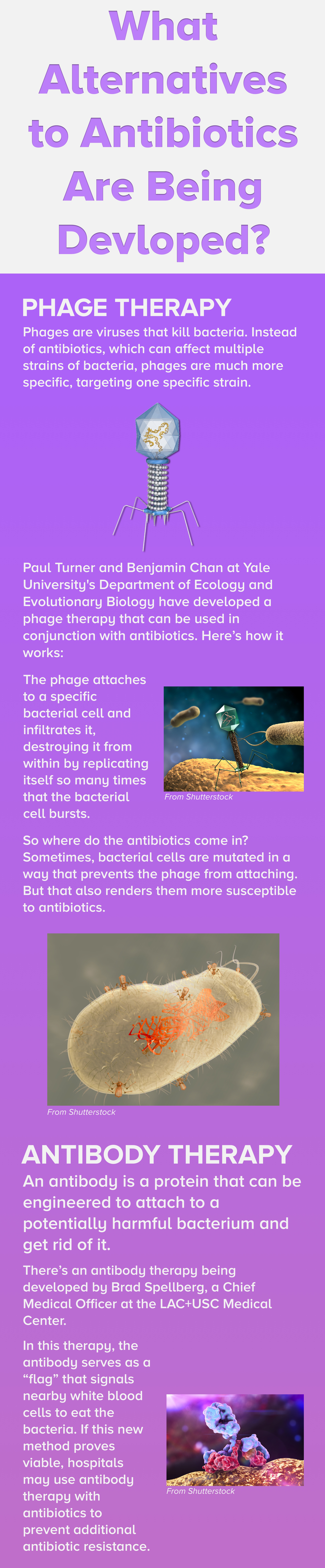
PAUL TURNER: All right. So we are capitalizing on the classic idea of phage therapy, where– this is an old idea that basically is gaining resurgence, where you use phages, which are bacteria specific viruses, to target the bacteria instead of chemical antibiotics.
So the trick is that phages, just like traditional antibiotics, the bacteria can gain resistance to them. So you have to use a strategy where you take the inevitability of evolution and you turn it in our favor in using the strategy. So that’s what we did.
IRA FLATOW: Because this is old. I mean, didn’t the Soviet Union sort of do a lot of work on this before World War II? Before we had miracle drugs?
PAUL TURNER: Yes. Interestingly, phage therapy dates back to near the discovery of phages, which is roughly 100 years ago. So the former Soviet Union and Eastern Bloc nations like Poland has been using phage therapy and trying to develop it for as long or longer as the US and Western nations have been developing traditional antibiotics.
IRA FLATOW: And it actually worked, didn’t it?
PAUL TURNER: Yes. It worked actually remarkably well. Many things held it back. Amazingly, some of it is that the primary results were not published in English. And also, we’re never translated. So ironically, some of the classic old and very convincing studies have just never really been read by Western physicians. And now that we’ve run out of options, we’re turning back to them.
IRA FLATOW: Details, details.
[LAUGHING]
IRA FLATOW: How about the human studies? How are they coming along?
PAUL TURNER: All right. So there are clinical trials that show that phage therapy in humans is both safe and effective. And ideas like ours that are new twists on the old idea, we would love to bring these to clinical trial soon, so we can make as many advancements as possible.
So our particular idea is that if you find a phage that targets a bacterium, and the way that it is targeting it is to control the virulence factor or the thing that the bacterium uses to cause disease, then you can actually exert selection pressure on the bacteria to either gain resistance to the phage and avoid it killing it, or they keep their antibiotic resistance.
So what we found is that they can’t do both at the same time. They’re painted into a corner. So we throw the phages at them and they must gain resistance. And at the same time, they simultaneously become more sensitive to antibiotics that are actually in our current drug arsenal. So it’s a way of kind of keeping those around.
IRA FLATOW: So they drop their guard in the first feint, and then you go in with the antibiotics.
PAUL TURNER: That’s true. They can’t do both things simultaneously. So they cannot be a jack of all trades. Instead we’ve found a way to turn old antibiotics that are continuing to be less and less efficacious and useful, and now we’re able to sort of use those still in combination with phages that exert the right kind of selection pressure.
IRA FLATOW: In your tests with humans, what kinds of tests have you been doing?
PAUL TURNER: Well so far we have approval from the US Food and Drug Administration to do what’s kind of described as compassionate care. So unfortunately, there very many individuals in the USA and elsewhere who have chronic bacterial infections, where these bacteria are resistant to antibiotics. They really don’t have any options left. So these individuals are very willing to undergo experimental therapy. And we got approval from the FDA to treat such individuals.
IRA FLATOW: I’m Ira Flatow. This is Science Friday from PRI, Public Radio International. Talking with research to combat those superbugs– talking with Brad Spellberg and Paul Turner. Brad, what do you think of this? When we did stories about bad bugs in the past it used to be all about MRSA or C. diff. It’s not such a problem anymore?
BRAD SPELLBERG: Well C. diff is a problem. It’s not an antibiotic resistance problem, per se. But it’s a side effect of antibiotic problem. It’s still around. There are probiotics that are being developed for C. diff that I think will become the standard of care for C. diff. In the next few years. MRSA is around.
Actually rates are declining across the United States and in many westernized countries for reasons we don’t fully understand. The real problem, though, for MRSA is when it gets into the blood or bone. We actually have had a ton of drugs developed to treat MRSA in the skin and in the lung. That’s no longer a huge antibiotic resistance problem.
IRA FLATOW: And what’s your idea about Paul Turner’s work with phages?
BRAD SPELLBERG: Well I’m quite familiar with phages. And I think that they are certainly an interesting alternative opportunity that we need to really study. I think that they need to be subjected to highly rigorous randomized control trials. One of the reasons they fell out of favor in the United States, as Paul alluded to, is there were not randomized control trials done, at least for invasive infections.
There were convincing trials done for like diarrheal illnesses. But for bloodstream infections or tissue infections, nobody had done the randomized control trial. Those are now being done, and I take my hat off to people like Paul that are pushing this technology forward. I think it is a promising alternative.
IRA FLATOW: So which kind of disease, Paul, do you think will be tried at first? What are you working on? What kind of illness?
PAUL TURNER: So currently we are focusing on multi-drug resistant pseudomonas aeruginosa, and that’s because this is a very commonly encountered bacterium. It’s in the human household, you see it in hospitals. And the point is it’s very dangerous for people like cystic fibrosis patients, severe burn victims, immune compromised individuals.
So many of your listeners may not have heard of such infections, but they are very, very common. So the idea is that we found a phage that was effective in targeting multi-drug resistance pseudomona aeruginosa, but we’re also searching for phages that work in a very similar way against other targets, such as klebsiella and pathogenic E. coli.
IRA FLATOW: I think what’s interesting as we’ve talked about this over the years– we’re now in our 25th year and I’ve been following phages– is that the phage which is a virus, there’s a virus that will attack every bacteria, isn’t there?
PAUL TURNER: I mean, yes. It seems that–
IRA FLATOW: Nature has a way to evolve that.
PAUL TURNER: That’s right. So I have argued, as many others have, that phages are actually the most numerous thing on the planet. So you can think of them as an almost endless pipeline for natural product discovery. If you go out and find a phage that has a useful application for many human problems that we suffer from, then I think we have many decades of promising research ahead to go find such phages and use them.
IRA FLATOW: And even as the bacteria evolved, the phages evolved with them to attack them.
PAUL TURNER: Yes.
IRA FLATOW: It’s amazing.
PAUL TURNER: They’ve been on the planet for billions of years alongside bacteria, so it’s not surprising that they can wage war with each other very effectively, and we can hopefully capitalize on that.
IRA FLATOW: Well I’m wishing you the best of luck. This is one of my pet subjects to follow all of these years. Paul Turner–
PAUL TURNER: Thank you.
IRA FLATOW: Your welcome. Paul Turner, Professor and Chair of the Department of Ecology and Evolutionary Biology at Yale University in New Haven. Thanks for joining us. We’re going to have more in antibiotic resistance. Stay with us. We’re going to talk more about antibiotics and how they travel through the feed lots, the waterways, and even vegetables have a– get into our systems just about everywhere. How they show up in your food, how they show up– well we’ll talk about it. Stay with us.
This is Science Friday. I’m Ira Flatow. Antibiotic resistance is our topic this hour with my guest Brad Spellberg, Chief Medical Officer at LAC+USC Medical Center in Los Angeles. I’d like to turn now to the issue of antibiotics and your burrito.
I’m talking about the antibiotics, of course, used in livestock, either to prevent and treat infection, or to simply fatten the animals up. Felicia Wu is the John A. Hannah distinguished professor in the Department of Food Science and Human Nutrition at Michigan State University in East Lansing. Welcome to Science Friday.
FELICIA WU: Thank you. It’s a pleasure to be here.
IRA FLATOW: You’re quite welcome, Dr. Wu. How does our use of antibiotics in agriculture compare to the amount we use, let’s say, in clinical settings?
FELICIA WU: Well the total amount of antibiotics used in animal agriculture is quite large. I think, perhaps, a more relevant question is what is the contribution of the use of antibiotics in animal culture versus in human medicine, to the problem of antibiotic resistance that we see today? And that is not a question that we really know how to answer yet.
IRA FLATOW: Why do you say that?
FELICIA WU: Well there’s many different– so sometimes when we do encounter a case of antibiotic resistance, it’s very difficult to state exactly how it was that the bacteria that caused the infection acquired resistance in the first place.
There’s actually a fairly large number of different pathways by which bacteria could have developed resistance. They could have developed it in the environment, which is one of the areas that I’m interested in working in. They could have developed it in through clinical routes, through medical routes, many different routes.
IRA FLATOW: Brad Spellberg, the idea that agricultural use could lead to more resistant bugs in health care settings, does that ring a bell with you?
BRAD SPELLBERG: Well I don’t even want to call it a debate. This has been a discussion that’s been going for years. To answer your question, Ira, four times as many antibiotics are purchased for use in livestock as for use in human beings in the United States. It is true that we cannot say definitively what proportion of resistant infections in humans is attributable to the use of antibiotics in animals. But we can say there is a contribution.
And so societally, rather than getting into a debate on the minutiae of exactly what proportion of this causes that, the point really is since we know it contributes, and that’s a serious societal negative or con, what is the pro that it offers society that should allow us to want to put up with that con?
And the pro really is that the people who use antibiotics in livestock believe that it allows them to make more money. So one group in society believes it can make more money by putting antibiotics into livestock, which does create a risk of harm to other people in society, why our society chooses to tolerate that remains unclear to me.
IRA FLATOW: Felicia, would you agree with that?
FELICIA WU: So those are interesting points as to the question. Yes, there certainly is. There are a number of economic reasons that animal producers do wish to use antibiotics in animal feed. And I’m going to go over the three main reasons.
One is for disease treatment, infectious disease treatment, to treat animals with infectious diseases just as with humans. The second is–
IRA FLATOW: Is that before– excuse me. Is that before or after they get the disease? Is that prophylactic, in other words?
FELICIA WU: Well so therapeutic use of antibiotics in animal operations can be both prophylactic, as well as after the fact. And so those are two reasons to use it, for disease treatment and disease prevention. And in addition thus far, in antibiotics in animal agriculture, have also been used for growth promotion. In order for the animals to grow more quickly, in order for them to be able to utilize their feed more efficiently, and thereby able to produce larger animals more quickly to create more profit.
IRA FLATOW: And when I read the fine print on the quote antibiotic free meat, it says they’re not given antibiotics for growth. But that doesn’t mean they’re still not getting a bunch of antibiotics for other things.
FELICIA WU: Exactly. And that is the way that the FDA policies on antibiotics used in animal feed has been going as well. That essentially, through the FDA Final Guidance 209 and 213, as well as their Veterinary Feeds Directive– or VFD– final rule, the idea behind these policies is that antibiotics that are important for human medical uses will be phased out for use in animal agriculture in terms of for the specific use of growth promotion and feed efficiency. They can still be used for therapeutic purposes, but that would be specifically under the supervision of licensed veterinarians.
IRA FLATOW: Brad what do you make of this argument by farmers? If they’re not allowed to use antibiotics to fatten up their animals, they won’t be able to compete economically with farmers abroad.
BRAD SPELLBERG: Yeah so there’s multiple points in agreement. Prophylaxis is not therapeutic. Nobody is arguing with the desire to treat sick animals. That’s perfectly legitimate use of an antibiotic. What is happening is people have finally begun to intellectually attack the concept of growth promotion, is people are just changing the name and they’re saying, “Oh well, it’s not growth promotion. That’s prophylaxis.”
Prophylaxis in almost no setting is acceptable in humans. In almost no setting should it be acceptable in animals. There are a few settings where there is an incredibly high short-term risk of infection where it makes sense. But so the answer to your most recent question, Ira, is that there have been multiple countries that have already banned growth promotion in routine prophylactic use of antibiotics.
And not only have their farmers not been harmed economically, they’ve expanded their herds after the band. And recent economic analyses are really suggesting that the historical growth promotional benefits of antibiotics may no longer be that economically impactful. We may be overestimating even the economic benefit to the farmers.
But I want to go back to this point. Should we, as a society, decide that a small economic advantage to a small group of people in our society is a reason we should accept creating a risk of harm to everyone else in society? Does that make sense from a societal policy perspective?
IRA FLATOW: Felicia, I’m pushing that question in your direction.
FELICIA WU: [LAUGHING] Well the advantage this is, as Doctor Spellberg pointed out, it’s not entirely clear. Because these FDA policies will not come into effect for about– for over about another half a year. These are going to come into effect in the beginning of 2017 and of the end of this year. So it’s entirely possible that we may find, as in the case with other countries, that the use of these antibiotics for therapeutic purposes and not for growth promotion may not have as much of a potential impact on the competitiveness of our animal product markets, as some may suspect.
We don’t know that for a fact yet. There are many people who are concerned about what would happen if, for example, we follow these particular policies, does that mean that we’re going to be less competitive on the global market for meat? There is that concern. But there are many companies, many food chains, for example, that are moving forward with purchases off antibiotic free meat. These include– Chipotle, Panera, Dunkin’ Donuts, and McDonald’s, for example, in the purchase of antibiotic-free chicken.
IRA FLATOW: Let me go to the phones. Our number, if you’d like to call in, 844-724-8255. Let’s go out to Kansas, Hutch, Kansas. Ben, hi, welcome to Science Friday.
BEN: Hi, how are you?
IRA FLATOW: Hi there.
BEN: Yes, I was listening and the gentleman speaker, he made the comment. I’m a farmer in a cow herd here in Kansas. It’s true that we do use and try to grow more beef by antibiotics and hormones and all that. But at the same token, with the booming population in the world, isn’t the fact that we can produce more meat for people in the world got a value that is more than the dollar?
IRA FLATOW: Brad, how would you answer that?
BRAD SPELLBERG: Well you know, again, I’m going to go back to countries that have banned routine growth promotion and routine prophylaxis have not seen declines in the herds. Denmark had a marked expansion in its pig production, for example, after they did the ban. A.
B, there are other ways to feed people. And C, there are harms. There are harms. And when patients die of resistant infections, and we can say, “Well I don’t know what proportion of those infections are caused by routine use of antibiotics.” But when 80% of our antibiotic use is in livestock, we have to acknowledge that we’re saying, “It’s OK that we know people will be harmed, because we don’t know how many and because some people are making money off it.” To me that equation does not add up.
IRA FLATOW: Ben in Kansas, do you have no market for– Felicia was talking about Chipotle, McDonald’s, other meat users now, going to antibiotic-free food. Do you have no market for people like that?
BEN: Oh, I have– I mean there’s a way to get a market for that. And you know, we have access to quite a few markets like that. And on our herd, we don’t– I mean it’s not just a shotgun approach where every animal gets treated. I mean, we keep animal health in our minds as much as our family’s health.
To where, let’s treat an animal when it needs to be treated and not at otherwise, because those antibiotics aren’t free and we are out here to make money. So we do consider costs that go into that animal versus costs that come out of that animal. So it’s not just a shotgun approach, that the whole industry is that way that I think it gets kind of lumped together.
BRAD SPELLBERG: And Ben, I think that’s an important point. Because again, nobody’s criticizing you for treating sick animals. Sick animals need to be treated. It sounds like you may not be using routine growth promotion, or routine prophylaxis, which is more of a shotgun approach.
IRA FLATOW: All right, thanks. Thanks for cluing us in, Ben. Good luck out in Kansas.
BEN: Good show.
IRA FLATOW: Thank you. Felicia, can antibiotics actually get into the flesh of an animal raised on antibiotic feed and can we eat that? Is it still active as an antibiotic?
FELICIA WU: Thankfully that is not a problem in the meat that we purchase in the United States. There’s actually a policy, by which, whenever a pharmaceutical company is going through the approval process for an antibiotic to be used in animal feed for the FDA, they have to demonstrate to the FDA that there’s no antibiotic residue that remains in the meat or other parts of the animal, like the liver or other specific tissues, for a certain period of time, which is called a withdrawal period. And in that withdrawal period, if the antibiotic residue was still in the animal then that animal cannot be taken to market. If it is taken to market within that period of time and there is antibiotic residue, then the producer actually faces a potential penalty. I’m Ira Flatow.
BRAD SPELLBERG: [INAUDIBLE].
IRA FLATOW: This is– wait, let me just– I’ll just jump in for a second– I’m Ira Flatow. This is Science Friday from PRI, Public Radio International. Talking about the hot topic of antibiotic resistance. Did you want to jump in there Brad?
BRAD SPELLBERG: Yes, sorry Ira. I just wanted to clarify. I think the real concern is not so much that there’s residual antibiotics in the meat. Their concern is during the butchering process, there is almost invariably some degree of contamination of bacteria in the meat. And those bacteria were exposed to antibiotics and therefore are often antibiotic resistant. So the concern is transmission of antibiotic resistant bacteria from the meat, from the excrement of the animals, which is used to fertilize crops, which can get into ground water, which has been shown possibly even to be spread in the airborne ways. So it’s more the spread of bacteria that are bred to be resistant by exposure to the antibiotics.
IRA FLATOW: And Dr. Wu–
FELICIA WU: And I–
IRA FLATOW: Go ahead.
FELICIA WU: I think that that’s a very interesting point, Dr. Spellberg. My area is in risk assessment. And our group is interested in that this problem actually has three different things that we should really be concerned about. One is the antibiotics.
The second, as you mentioned, is the antibiotic resistance bacteria. And the third is antibiotic resistance genes, and how they might spread in the environment and how we might be exposed to them through various different routes and pathways of exposure. Each of these problems has to be treated a bit differently, actually, for the examples that you raise.
Are we really concerned if there are antibiotics in the meat? Well that isn’t really a problem here in the United States, but what about antibiotic resistant bacteria? What about the genes? What’s the likelihood that if we accidentally consume these antibiotic resistant bacteria, that other bacteria in our gut might acquire resistance? These are all interesting risk assessment questions. And these are actually fairly separate risk assessment problems.
IRA FLATOW: Can they, Dr. Wu, get into our fruits and vegetables?
FELICIA WU: Certainly.
IRA FLATOW: Think the bacteria can?
FELICIA WU: Oh, OK. Yes, yes. So several studies have shown that indeed, if there are antibiotics in the environment, if they are in irrigated water, then certain crops can take up the antibiotics. A recent study showed, for example, that carrots and lettuce can actually take up both tetracycline and amoxicillin, if it’s in the irrigated water. So that is another potential source of exposure to antibiotics inadvertently through produce.
IRA FLATOW: Are they still viable when we eat them?
FELICIA WU: So it’s a good question. They may possibly be viable. The question has to do with the dose. So are these doses likely to have effects of killing bacteria? We don’t really know. And are they at doses that may put pressure on bacteria to lead to resistance? That’s another thing that we do not know yet.
IRA FLATOW: I understand, you mentioned before, that they can even get into the air? Antibiotics?
FELICIA WU: Yes, that’s correct.
IRA FLATOW: How do they do that? We could be breathing them in?
FELICIA WU: Well there was a very interesting study that just came out last year, that studied how particulate matter in the air can actually transport the antibiotics– antibiotic resistant bacteria and antibiotic resistance genes, through wind dispersal. So this study showed that within roughly 10-20 meters from cattle feed yards, if they were to take samples of the area and find the particular matter, there they would also find the antibiotic resistance genes, the bacteria, and the antibiotics themselves.
IRA FLATOW: Another way to spread them. Wow.
FELICIA WU: Yes.
IRA FLATOW: Wow. I can’t– I have nowhere to go after that. [LAUGHING] That’s mind blowing enough. Felicia Wu, John A. Hannah, the distinguished professor in the Department of Food Science and Human Nutrition at the Michigan State University in East Lansing. Brad Spellberg, Chief Medical Officer at the LAC+USC Medical Center in Los Angeles. Thank you both for joining us today.
BRAD SPELLBERG: Thank you, Ira.
FELICIA WU: Thank you.
Copyright © 2016 Science Friday Initiative. All rights reserved. Science Friday transcripts are produced on a tight deadline by 3Play Media. Fidelity to the original aired/published audio or video file might vary, and text might be updated or amended in the future. For the authoritative record of ScienceFriday’s programming, please visit the original aired/published recording. For terms of use and more information, visit our policies pages at http://www.sciencefriday.com/about/policies.
Christopher Intagliata was Science Friday’s senior producer. He once served as a prop in an optical illusion and speaks passable Ira Flatowese.
D. Peterschmidt is a producer, host of the podcast Universe of Art, and composes music for Science Friday’s podcasts. Their D&D character is a clumsy bard named Chip Chap Chopman.