3-D Printing Living Cells
8:42 minutes
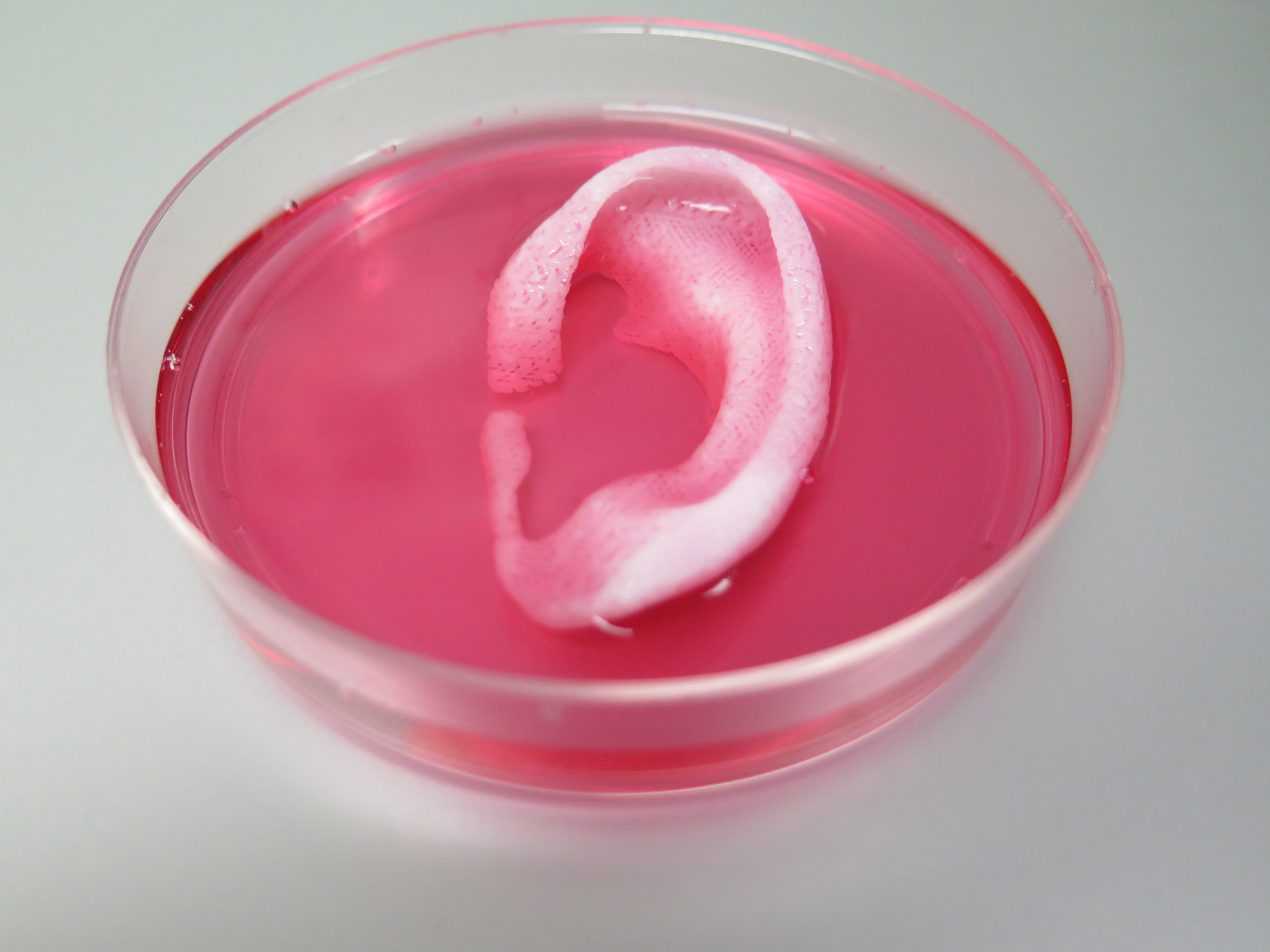
The use of 3-D printers has opened up the possibility of on-demand implants, prosthetics, and medical devices. This week, scientists reported that they were able to 3-D-print the first stable ear, bone, and muscle structures out of living cells and implant them in mice. The results were published in Nature Biotechnology. Anthony Atala, the director of the Wake Forest Institute for Regenerative Medicine and an author on that paper, describes the challenges of 3-D printing living cells and how the technology could be used in bioengineering body parts.
Anthony Atala is director of the Wake Forest Institute for Regenerative Medicine at Wake Forest University in Winston-Salem, North Carolina.
IRA FLATOW: Two years ago doctors at the University of Michigan were able to 3D print a trachea for a 16-month-old windpipe. That device saved the baby’s life. And that trachea was made out of hard plastic. But what if we could print body parts that are more true to real life? Made up of living cells. Doctors have been tinkering with 3D printers to regenerate tissue. And so far the results have been mixed. Well this week researchers reported printing out ear, muscle, and bone tissue, and implanting them in mice. The results were published in the journal Nature Biotechnology. My next guest is an author on that paper. Anthony Atala is the director of Wake Forest Institute for Regenerative Medicine. That’s at, of course, Wake Forest University at Winston-Salem, North Carolina. Welcome to Science Friday.
ANTHONY ATALA: Nice to be with you today.
IRA FLATOW: Is the 3D printer that you use just like the one that people use to create jewelry and prototypes and stuff like that?
ANTHONY ATALA: Well, you know the concept is very similar, Ira. It’s actually a very similar concept because at the end of the process you’re really printing structures. But the actual process, of course, can be very damaging to the cells. So you can print plastics and metals. The printing techniques are similar. But the strategy is to make sure that the cells don’t get damaged during the printing process.
IRA FLATOW: Tell us exactly how you print it.
ANTHONY ATALA: Well, it’s actually a system we start to develop about 10 years ago. And it was basically based on the concept that you want to make sure that the cells go down to the nozzle– the printing nozzle– with actual survival. So the nozzles have to be very thin to be able to print precisely where you need the cells. But it’s also important to make sure they survive. So we did that using various strategies. The main one was to make sure that we could lay down the cells and they would retain their structural integrity as you print them.
IRA FLATOW: Tell us how you give– yeah, because you need to have them pretty sturdy. How do you give them that structural integrity?
ANTHONY ATALA: That’s a great question. Because, you know, the process is– you want to make sure the cells go down to the nozzle as a liquid. But the moment it hits the surface you want to make sure it’s more like a gelatin. Because otherwise it’ll be just the blotch of fluid coming down. So you want it to basically hit the surface as a gelatin. And then you want it to harden as a gummy bear. And the whole process needs to happen at a time when the whole construct retains a structure. So we print a shell, if you will, around the structure. And this hard shell keeps everything in place. And once the construct is completed– once the printing is completed– we can then use water to dissolve the outer shell.
IRA FLATOW: So you have a living tissue made out of the cells, like an ear, or its muscle cells. And how do you get the blood– you have to get all that stuff to work from the body with it. How do you do that?
ANTHONY ATALA: Another good point. Basically the challenge, of course, is to make sure that these things survive. You know, not just that you have the cells go through one be alive, but also to make sure that they remain alive and that they get fed. And the problem has been, of course with nature given that there’s a maximum distance of the fusion of nutrients of about 0.12 to 0.2 millimeters. That is, we can print small structures– about 0.1 to 0.2 millimeters. And they’re going to be fed just by the surrounding area. But anything larger– the cells in the center will not survive. So what we did is we basically built highways, micro channels or highways, into the central portion of the structure so the nutrients could get right in.
IRA FLATOW: And so when you attach these– and you’re attaching them to your lab animals. Do they just take up and grow the right cells in the right place?
ANTHONY ATALA: Well, the cells are already in place. But then when we implant them, experimentally, what happens in the body is that the nutrients come in. You have that micro channel, or that highway system, for the nutrients to come in and help the tissue survive. But then at the same time the blood vessels from the body will start sprouting it’s capillaries, or it’s tentacles if you will, into the tissue and it replaces the micro channels with the body’s own blood vessels.
IRA FLATOW: I’m Ira Flatow. This is Science Friday from PRI, Public Radio international. Talking with Anthony Atala of Wake Forest. So I understand that the ear cells that you tried came from a rabbit and you implanted it into a mouse. And my question is– why wasn’t it rejected? It being from a different kind of animal, or another animal altogether.
ANTHONY ATALA: Yeah, that’s a good– basically we use these mice that basically don’t have a sophisticated immune system. So you can use other cell types that go in there. But of course we’re doing this with plans to do this in patients. And if we were to create a patient the concept would be that we would take a small piece of tissue from the patient and we would then grow the cells outside the body– very small piece, less than half the size of a postage stamp– we would then grow the cells outside the body and then place those cells into the printing cartridges for the printing process.
IRA FLATOW: Yeah, well you–
ANTHONY ATALA: And then we would–
IRA FLATOW: Yeah, and then you’d–
ANTHONY ATALA: Oh, sorry.
IRA FLATOW: No, I’m sorry. I didn’t mean to interrupt. And I imagine you were going to say you did that and put it back in the patient?
ANTHONY ATALA: Exactly. Then we put it back into the same patient and avoiding rejection.
IRA FLATOW: Well that is the $64 question now, right? When are you going to do that with people?
ANTHONY ATALA: Certainly that’s why we’re doing this work. And we are currently doing the safety trial, safety studies, pre-clinical studies that will hopefully lead us to our patient experience in the future.
IRA FLATOW: And how soon do you think we can see that?
ANTHONY ATALA: Well, in science, as you know, it’s always hard to predict when these things actually can get to patients because we do have the regulatory process to go through. But definitely that’s something we’re working on very hard to make sure that we can get that to patients in the future.
IRA FLATOW: You know, something that we always wondered about– remember back in the ’90s, we all remember that human ear that was growing on the back of a rat and–
ANTHONY ATALA: Yes.
IRA FLATOW: There was a lot of promise. Now we’re 20 years– almost 20 years later. How will 3D printed structures avoid the fate of the rat, I imagine, which is still not alive anymore?
ANTHONY ATALA: That’s exactly right. And, you know, the challenge, of course, was that you didn’t have the right blood vessel supply going into it. And this has been a challenge now for quite some time. How do you actually make sure that these tissues survive? So it’s easy to print small structures. But when you print large structures you really do need that blood vessel supply. And that’s what’s really prevented some of these technologies from progressing So by having these micro channels you can really make that work. These constructs do get the vascularized and innervated once inside the body.
IRA FLATOW: What do you think the first body part will be tried out on a person?
ANTHONY ATALA: Well, there’s a different level of complexity. All tissues are complex but the lease complex are flat structures, such as skin. They’re flat. Mostly one major cell type. Tubular structures are the second level of complexity, like blood vessels. Two major cell types is tubular, not flat. Hollow non-tubular organs are the third level of complexity, like the stomach or the bladder. Architecture is more complex, more interaction with other organs. And finally, the most complex are the solid organs, like the liver, the heart, the lung. And we’re going to follow that same path in terms of least complex to most complex as we take these things into the clinic.
IRA FLATOW: Well, we wish you good luck with this. This is something we’ve been, as I say, starting with the year on the rat– something we’ve all been following for years and waiting for some good positive results. So maybe we’ll be hearing from you pretty soon on something, Anthony.
ANTHONY ATALA: Well, hopefully so. It’ll be a number years but hopefully we’ll get there.
IRA FLATOW: All right. We’ll be here for you Anthony Atala, Director of Wake Forest Institute for Regenerative Medicine. That’s of course at Wake Forest University in Winston-Salem, North Carolina.
Copyright © 2016 Science Friday Initiative. All rights reserved. Science Friday transcripts are produced on a tight deadline by 3Play Media. Fidelity to the original aired/published audio or video file might vary, and text might be updated or amended in the future. For the authoritative record of ScienceFriday’s programming, please visit the original aired/published recording. For terms of use and more information, visit our policies pages at http://www.sciencefriday.com/about/policies.
Alexa Lim was a senior producer for Science Friday. Her favorite stories involve space, sound, and strange animal discoveries.