On The Frontier Of An Alzheimer’s Cure
16:58 minutes
Alzheimer’s disease is known for inflicting devastating declines in memory and cognitive function. Researchers are on the hunt for treatments are taking a number of approaches to slowing or preventing the neurodegenerative disease, including immune therapy, lifestyle changes, and targeting sticky buildups of proteins called amyloid beta.
But at MIT, scientists have been trying something else: a combination of flashing strobe lights and a clicking sound* played at 40 times per second, for just an hour a day. They wrote in the journal Cell earlier this month that mice given this treatment for a week showed significant reductions in Alzheimer’s signature brain changes—accumulations of amyloid plaques and tangles of a protein called tau (see videos below). Furthermore, the mice had marked improvements in cognition, memory, and learning.
But the big question in Alzheimer’s research: Can improvements in brains of mice translate to human subjects? Dr. Li-Huei Tsai, an author on the research, talks with Ira, and Wake Forest Medical School neuroscientist Dr. Shannon Macauley, who was not involved in the research, discusses how to take promising research of all kinds to the next level.
Plus, why the failure of the drug Aducanumab in clinical trials this week spells bad news for the amyloid beta approach to Alzheimer’s treatment.
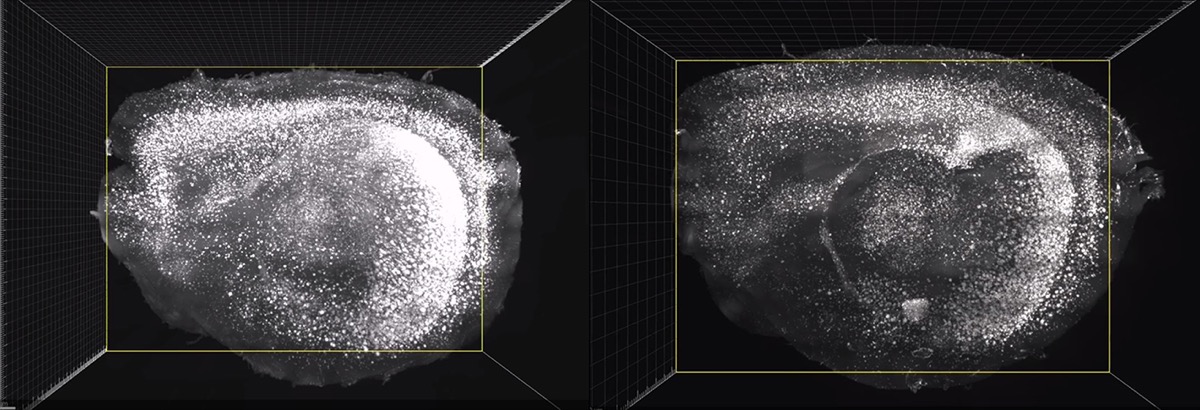
View videos of mice brain scans with and without the light and sound treatment, and of the microglia ganging around amyloid plaque.
This mouse has been treated with light and sound therapy for its Alzheimer’s. After the treatment, the brain had less amyloid. Credit: Picower Institute for Learning and Memory/MIT
Brain scans of an untreated mouse with Alzheimer’s shows more amyloid presence. Credit: Picower Institute for Learning and Memory/MIT
Microglia (stained green) gang up on an amyloid beta plaque in the cortex of a mouse. Credit: Picower Institute for Learning and Memory/MIT
*Editor’s Note: The clip of the clicking sound that appears in Science Friday’s broadcast on 3/22/19 was distorted due to audio compression. We’ve updated the embedded audio on this page on 4/10/19 to provide a more accurate clip of what the mice heard during the study. However, the researchers note that the sound is still not an exact representation what they used in the study due to audio compression.
Invest in quality science journalism by making a donation to Science Friday.
Dr. Li-Huei Tsai is a professor of Neuroscience and the Director of the Picower Institute for Learning and Memory at the Massachusetts Institute of Technology in Cambridge, Massachusetts.
Shannon Macauley is an assistant professor of Gerontology And Geriatric Medicine and a member of the Alzheimer’s Disease Research Center at the Wake Forest School of Medicine in Winston Salem, North Carolina.
IRA FLATOW: This is Science Friday. I’m Ira Flatow.
Chances are we all know someone with dementia– like Alzheimer’s disease. And we are all worried, at least in the back of our minds, about our chances of falling victim to it. Because we know there is still no cure for it, and we eagerly listen for any hopeful news about advances in research– because so far Alzheimer’s treatments have not really worked. Well, we now have some of that hopeful news from a team from MIT that has been thinking differently.
Here you go– imagine a therapy you are sat down with just a flashing light and a clicking sound– all pulsating at 40 times a second, for just an hour a day. If you’re a mouse engineered to model Alzheimer’s, it turns out this very alternative therapy has some striking results. Cognitive function improves. Memory and learning improves. These pesky, sticky amyloid plaques clear up, and your brain produces a very specific pattern of neuron firing called gamma waves.
As Alzheimer’s researchers know too well, translating research results from mice to human brains can be a tall order. So what’s next for this light and sound idea? Where does it fit with other things researchers are trying in the quest to reduce the devastating effects of Alzheimer’s disease?
Here to explain her research is Li-Huei Tsai, cognitive neuroscientist and director of MIT’s Picower Institute for Learning and Memory. She’s one of the authors of this study published in the journal Cell.
Welcome, Dr. Tsai.
LI-HUEI TSAI: Thank you.
IRA FLATOW: And also with us is Dr. Shannon Macauley, assistant professor of gerontology and geriatric medicine and a member of the Alzheimer’s Disease Research Center at Wake Forest medical school in Winston-Salem, North Carolina. Welcome, Dr. Macauley.
SHANNON MACAULEY: Thank you.
IRA FLATOW: Let’s talk, Dr. Tsai– first, about this study you did. What made you think sound and light could have any effect on Alzheimer’s symptoms?
LI-HUEI TSAI: [LAUGHS] Yeah, this is a very good question. So when we look at Alzheimer’s disease, we kind of look at it from a different angle. So we all know that there are plaques and tangles and cell loss, but few people look into the brain waves. And we found that these brain waves– especially at the 40 Hertz frequency– we call it gamma waves– they are impaired in a very early stage of the disease. So we ask, what will happen if we increase the 40 Hertz gamma waves in the brain of Alzheimer’s disease mouse models.
IRA FLATOW: Mm-hmm. So you sat the mice down with the 40 Hertz waves of sound and light at the same time, just flashing lights in front of their eyes and sounds through their ears?
LI-HUEI TSAI: Exactly. So really much to our surprise, we found that if we just flash 40 Hertz light and present the 40 Hertz sound to the mice, then their brains started to produce these gamma waves very robustly. So we realized that we can easily use this very non-invasive approach to induce gamma waves in the brain.
IRA FLATOW: Mm-hmm. Just to let our listeners hear what those clicks sounded like– just a warning, your radio is broken when you hear this–
[CLICKING SOUNDS]
Wow, those clicks– plus the flashing light– they were able to clear out the brain of amyloid and tau and all these physical indicators of Alzheimer’s in the brain?
LI-HUEI TSAI: You know it sounds crazy, I know.
SHANNON MACAULEY: Mm-hmm.
LI-HUEI TSAI: But it just obtains these striking results every time we do it.
IRA FLATOW: And how long does it last for?
LI-HUEI TSAI: Yeah, this is a very good question. So far, we believe that we do have to do it continuously. We presented the light and sound to mice one hour a day, every day, and we started to see these effects after about a week. And if we do it longer, we see even a better effect.
IRA FLATOW: Mm-hmm. And it affected their learning ability and their memory and help them in those ways?
LI-HUEI TSAI: Indeed. Yeah, we not only saw reduction of plaques and tangles. We also see that their memory becomes better, and they learn better.
IRA FLATOW: Huh. It sounds almost too good to be true. [LAUGHS]
LI-HUEI TSAI: I understand.
IRA FLATOW: [LAUGHS]
LI-HUEI TSAI: I understand. [LAUGHS]
IRA FLATOW: Well, Dr. Macauley, what does the rest of the Alzheimer’s research community think about this work? Is it too out there?
SHANNON MACAULEY: You know, I think this is a really highly innovative approach, and it’s creative– and the findings, I agree, are fairly provocative. Right? You know– to just think that these series of flashes and auditory clicks could clear plaques and tangles and restore cognitive function is exciting but a little bit out there.
But if you start to think about the pathogenesis of Alzheimer’s disease and the fact that the Alzheimer’s diseased brain has a problem with hyperexcitability– we know that neurons aren’t functioning properly– we know networks are impaired– there’s a great deal of synaptic dysfunction. And so if this approach can almost provide the brain with a reset button to normalize brain function, it could be a possible treatment for Alzheimer’s disease.
So the findings are absolutely provocative, I agree, but they’re very convincing. And the fact that there was this stimulation of microglial clearance– so these are your garbagemen of your brain that can clear away debris– and that Dr. Tsai’s work shows that there is this increase in this ability to clear bad proteins from the brain– really does suggest that it has traction. And I’m very excited to see it translated up to humans.
IRA FLATOW: Now, let’s talk about that, Dr. Tsai. When do we have human trials of this? Because you’re not really doing anything invasive, you know? When do we start a clinical trial?
LI-HUEI TSAI: Yeah, it’s a very good question. I’m very excited about this approach, precisely because this is so non-invasive. And in mice, we know that even after we presented the light and sound every day for months and months, these mice look completely healthy. They get better and better with their memory and learning, and they seem very healthy. There’s no deleterious effects, as far as we can tell.
So I think that– with this kind of very safe and very non-invasive approach– I think it will be relatively straightforward to translate to humans. But really the $100 million question is whether the human would respond similarly to how the mice responded to this treatment.
IRA FLATOW: Yeah. But you have two very simple things– you have some flashing lights, and you have this 40 Hertz sound. What’s to prevent any physician from just setting this up in his or her own office to try it out?
LI-HUEI TSAI: So that’s why here, at MIT, we actually started some very early feasibility and compliance kind of study in human subjects. Primarily, we just really want to figure out a good condition and a good device to induce gamma waves in human subjects, so that we can make sure that when people do it, we have the best condition for them.
IRA FLATOW: Mm-hmm. Dr. Macauley, there was some other big Alzheimer’s news this week, and that was– Biogen drug aducanumab has officially been pulled out of phase III clinical trials. There were, as you know, lots of hopes riding on that drug. Right?
SHANNON MACAULEY: Right. Yeah, absolutely. So I think we just found that out Thursday– that aducanumab– they decided to stop their phase III clinical trial. And that was a pretty big blow to the field. And again, as we try to work very hard to get something to patients and caregivers, it’s a major setback for us.
Reports, even within the last year, have suggested that this immunotherapy that targets amyloid– one of the earliest changes in the Alzheimer’s disease cascade, or thought to be– it shows that it can bind the toxic forms of amyloid– it can remove amyloid from the brain– and even showed some promise on cognitive improvement, which is kind of our holy grail for trying to get a therapy to market.
So the fact that they decided to stop this trial– and the good news was it’s not because of a safety issue– but unfortunately, the bad news is it was because they didn’t think they could reach their primary endpoints for slowing cognitive decline. So yeah, that’s a pretty big blow to us, at this point.
IRA FLATOW: Is there any thought that we might be aiming at the wrong targets here?
SHANNON MACAULEY: Absolutely. So I think one thing that we have to keep in mind, with all of these amyloid-based therapies, is we’ve learned so much in the recent decades about how Alzheimer’s disease progresses in humans. And the fact that amyloid does seem to be one of the first things that changes– and you do need to see amyloid and tangles at autopsy to give a diagnosis of Alzheimer’s disease.
So I think originally the idea that if we could stop the amyloid from accumulating or remove it after it’s accumulated from the brain– as a way to stop Alzheimer’s– was sound. But I think what we’re finding now is after all of these trials– where we’ve made some great, I think, improvements in clinical trial designs and the antibodies and what type of amyloid beta we’re targeting– we still aren’t seeing that holy grail effect on cognition.
And we need to change our approach to look at different things that go wrong in AD. We know, when individuals start to go through cognitive impairment and cognitive changes, that it’s tau that’s rising– the protein that’s found in neurofibrillary tangles. We see this huge neuroinflammatory response– changes in metabolism. And so there’s a whole host of other targets that I think we need to go full force on in order to treat this disease.
IRA FLATOW: Dr. Tsai, the fact that your study, with the light and the sound, was effective in removing both the amyloid and the tau– do you think that was what might have made a difference?
LI-HUEI TSAI: You know, I think our approach– still yet to be tested in humans. But I agree with Dr. Macauley. I think at this point we still, simply, don’t know enough about the disease. And the disease, clearly, is very complex, and probably people get Alzheimer’s disease from different etiology.
And so I think that, in addition to these individual molecules and genes, we really need to also look at the brain as a whole systems. The brain functions like a computer, so we need to look at the circuits. We need to look at the network and figure out what’s wrong with the network and how can we change the whole network to change the state of the brain.
IRA FLATOW: Interesting.
This is Science Friday from WNYC Studios. We’re talking about Alzheimer’s disease here.
Dr. Macauley, we talk about– in this business– about changes happening so many years before we actually see symptoms.
SHANNON MACAULEY: Right.
IRA FLATOW: Do you hold out an idea that there might be early detection– getting even easier? And if we do detect it earlier, does it make treatment any easier or more effective?
SHANNON MACAULEY: Absolutely. I think you hit the nail on the head. Again, over the last decade, we’ve seen a tremendous jump in the technologies we can use to actually stage this disease. We have new neuroimaging biomarkers that can see plaques in the brain and can see tau accumulating in the brain. We have CSF biomarkers that, again, can look not only at tau and ABeta levels, but can also get at changes in neuronal integrity. And now, even within the last couple years, we’re developing serum-based– so blood-based biomarkers of disease.
And so what this is allowing us to do is really get a composite of what’s happening and as Dr. Tsai says, move beyond just amyloid and tau. Right? So we can look at neuronal integrity– when do these networks– their network activity become aberrant– or neuronal loss starts to happen. And that might guide when we intervene and what we intervene with.
So I think the big thing right now is really understanding how the human clinical course of disease happens and understand the heterogeneity within that data set as well.
IRA FLATOW: Hmm. We had Dr. Eric Topol on just now, talking about his ideas about deep medicine and using artificial intelligence. Do you think there is a use for artificial intelligence, going through all the data of patients?
SHANNON MACAULEY: Absolutely. I mean, I think that we’re creating– from genetics to cellular function– I mean, to the point we’re getting single-cell RNA data– it’s such massive data sets out there that there needs to become a way that we can look at a signature for Alzheimer’s disease– and one that incorporates function.
Again, what Dr. Tsai says is that we really need to make sure that we’re bringing this back to preserving cognition, network connectivity– and also an interaction between the brain, the vasculature, metabolism, and all of this. So yeah, I do think that we need to start to think about this in terms of big data and [INAUDIBLE].
IRA FLATOW: Dr. Tsai, where do you go–
LI-HUEI TSAI: Yeah, exactly.
IRA FLATOW: I’m sorry– where do you go from here with your work with the mice now?
LI-HUEI TSAI: Yeah, so with the mice, we’ve so far demonstrated that both light and sound can induce gamma waves. But we have more senses– capacities– so we would like to see whether by other sensory stimulation– such as smell or tactile stimulation– that can also induce gamma waves. And also, we would like to further understand how this works.
So as Dr. Macauley said, we show that the microglia– the brain’s immune cells– respond robustly to these gamma waves. And they become much more active. They restore their normal function to clear the amyloid and presumably other toxic waste. And we also show in our Cell paper that the blood vessels in the brain also respond.
IRA FLATOW: I–
LI-HUEI TSAI: Um–
IRA FLATOW: I’m going to have to leave it there and have you back and talk about the rest of it, because that’s very exciting, Dr. Tsai.
I want to thank Dr. Li-Huei Tsai and Dr. Shannon Macauley for talking about their work on Alzheimer’s disease.
Copyright © 2019 Science Friday Initiative. All rights reserved. Science Friday transcripts are produced on a tight deadline by 3Play Media. Fidelity to the original aired/published audio or video file might vary, and text might be updated or amended in the future. For the authoritative record of Science Friday’s programming, please visit the original aired/published recording. For terms of use and more information, visit our policies pages at http://www.sciencefriday.com/about/policies/
Christie Taylor was a producer for Science Friday. Her days involved diligent research, too many phone calls for an introvert, and asking scientists if they have any audio of that narwhal heartbeat.
Ira Flatow is the founder and host of Science Friday. His green thumb has revived many an office plant at death’s door.