Twisted Science: Tying The Strongest Molecular Knot
7:56 minutes
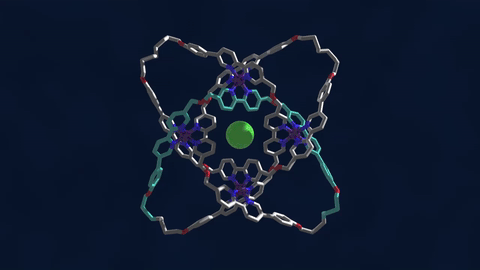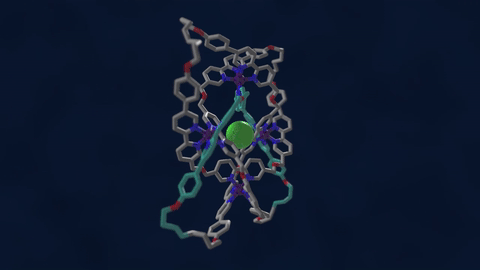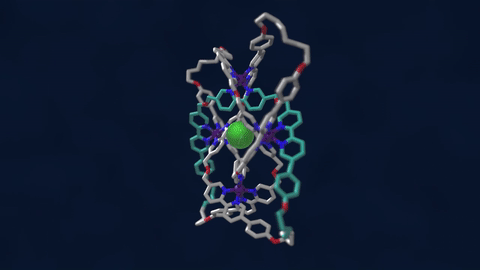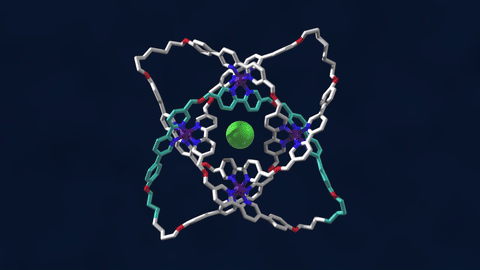
The bowline, clove hitch, and square are knots familiar to campers who might need to, say, tie down a tent with rope. But there are naturally occurring knots, too—ones that can be found on the molecular level in DNA and in protein strands. Over six different knot patterns have been identified. Reporting in the journal Science, researchers synthetically made the tightest molecular knot using metal ions, achieving eight different crossings in the structure. Chemist David Leigh, an author on the paper, discusses how molecular knots could be used to create stronger materials and molecular machines.
David Leigh is a professor of chemistry at the University of Manchester in Manchester, United Kingdom.
JOHN DANKOSKY: This is Science Friday, I’m John Dankosky, in for Ira Flatow. There’s an unseen process that probably happens to you every day. You take your ear buds and you put them in your bag, and when you pull them out later, they’ve become a knotted mess. How that happens is a mystery. We’re not going to solve that today.
You tie your shoes, you wrap a present, you go camping. We may think we know a lot of knots, but did you know that there are more than six billion different kinds? Many of these twisted patterns often occur naturally on the molecular scale. Strands of DNA and protein are packaged into these orderly tangles.
Now scientists have created the tightest molecular knot using chemistry. The results were published this week in the Journal of Science. My next guest is an author on that paper and here to tell us how you tie a molecule into knots. David Lee is a professor of chemistry at the University of Manchester, in Manchester, United Kingdom. David, welcome to Science Friday.
DAVID LEE: Thanks. Thanks for having me on the show.
JOHN DANKOSKY: So first of all, explain what exactly a knot is.
DAVID LEE: So in mathematical terms, a knot is a closed loop that’s got entanglements in it. So the knots that we tie in shoe laces and that Boy Scouts tie, those are open knots in terms of mathematics. They’re still knots, but if you close the end, you get a knot as a mathematician would understand it.
JOHN DANKOSKY: OK. So tell us about the difference here with molecular knots. Explain what a molecular knot is.
DAVID LEE: So a molecular knot is just where you take a molecule and you tie it into a knot. And that’s what we’ve been doing in our laboratory, learning how to do that.
JOHN DANKOSKY: OK. You’ve been learning how to do it. Explain it to us. How do you do this?
DAVID LEE: Well, the strands that we’re knotting are so small that you can’t grab the ends and mechanically tie them like you would a shoelace, say. So instead, we use chemistry and a process called self-assembly. So we mix together organic building blocks with metal ions and chloride ions in a solution, and these metal ions are sticky in particular directions, and the organic building blocks wrap around the metal ions, forming crossing points in the right places that we need, just like happens in knitting. And then, once all the crossing points are formed, the ends of these building blocks are then fused together by a catalyst to close the loop and form the complete knot.
JOHN DANKOSKY: And why is this important to do? Why are you tying these molecular knots?
DAVID LEE: Well, there’s many reasons. As you pointed out, that knots in molecules actually occur in biology, in DNA, in proteins, and they form spontaneously in polymer chains. But as every Boy Scout knows, different sorts of knots have different characteristics, which make them more or less useful for particular tasks. And knots should be just as important, versatile, and useful in the molecular world as they are in our everyday world. But we can’t exploit those things until we learn how to tie them.
JOHN DANKOSKY: So explain some of the exploitations. What are the things that you would do with molecular knots, especially ones of the type we’re talking about here, tied very, very tightly?
DAVID LEE: Well, knotting and it’s counterpart, weaving, have always led to break through kinds of technologies. So even for prehistoric man, knotting allowed him to make tools, such as fishing nets, and axes with blades tied to handles, and how to weave fabrics to keep him warm. So it should be possible to use knots to do similar sorts of things at the molecular level, to make new kinds of materials which have got new sorts of properties or beneficial properties.
So in our everyday world, we know the benefit of weaving fabric, so you get materials that can stretch in different directions, hold their shape, and they’re light, strong, and flexible. Hopefully, we’ll be able to use the same concepts that we’re learning about how to know molecular strands. We’ll be able to use that to weave molecular strands– they’re very similar concepts– and use that to make plastics with similarly advantageous properties.
JOHN DANKOSKY: Well, let’s talk about those properties. When you knot a molecule, it has a different structure. But what different properties does it have then?
DAVID LEE: Well, again, just like in weaving, perhaps you can get beneficial properties that make it more flexible and stronger for the same sort of– or lighter for the same sort of strength. So for example, take care Kevlar, which is a very strong plastic used in bulletproof vests, and body armor, and also car brakes, and so on. So its chemical structures is actually lots of tiny, straight molecular rods that pack together like pencils stuffed in a pencil case. So if we could weave molecular strands into molecular fabrics, maybe we’ll be able to get the same sort of strength as those kinds of materials, but with a much lighter and more flexible material.
JOHN DANKOSKY: So we lead by saying that you’ve created the tightest known molecular knot with eight crossings. So tell us about this, how this is created, and the strength that it gets from this very, very tight compact knot.
DAVID LEE: Yeah, so these strands that we’re making are really small. They’re just half a nanometer cross. That’s less than a millionth of a millimeter, or 10,000 times thinner than a human hair. So we’re talking about really tiny kinds of structures here.
And the loop, the strand of the knot is just 192 atoms long. It’s got eight crossings in it, so that’s just 24 atoms for every crossing that occurs in the knot. That makes it the tightest knotted physical structure known. And we’re interested, now, to finding out how that actually affects its physical and its chemical properties, because again, as every fisherman knows, actually tying knots in a strand can actually make it weaker. It’s the weakest part of a knotted strand. But if we are able to weave strands, conversely, that can make a material very strong.
JOHN DANKOSKY: So why is it that with these six billion known knots, it’s so difficult to synthesize these? We’ve only been able to come up with a few. Obviously, they’re very, very small. What makes this difficult?
DAVID LEE: Yeah. It’s about being able to gain control. It’s being able to control whether the crossings go under and over in exactly the right sort of ways, and it’s learning how to do that, which has been difficult. But now, we’re getting to be able to make more complex knots than previously possible. And using the techniques that we’ve learned, we’re now able to braid actual strings, just like one braids a child hair. We are beginning to braid molecular threads, and that allows us to access many more of these sorts of knots than has previously been possible.
JOHN DANKOSKY: What’s the next exciting thing you’re working on?
DAVID LEE: So just because we can tie knots, doesn’t mean that we can immediately go to weaving. So just because I can tie my shoe laces, doesn’t mean that I can knit a Christmas jumper. So we’ve really got to learn how to be able to connect the knots or extrapolate from the knots to weaving the sort of weaves that we need to make two dimensional materials, just like the fabrics that we have in our everyday world.
JOHN DANKOSKY: Well, David Lee, thank you for this interesting knotty conversation. I really appreciate it.
DAVID LEE: Thanks a lot.
JOHN DANKOSKY: David Lee is a professor of chemistry at the University of Manchester, in Manchester, UK.
Copyright © 2016 Science Friday Initiative. All rights reserved. Science Friday transcripts are produced on a tight deadline by 3Play Media. Fidelity to the original aired/published audio or video file might vary, and text might be updated or amended in the future. For the authoritative record of ScienceFriday’s programming, please visit the original aired/published recording. For terms of use and more information, visit our policies pages at http://www.sciencefriday.com/about/policies/
Alexa Lim was a senior producer for Science Friday. Her favorite stories involve space, sound, and strange animal discoveries.