Pulling Water From Thin Air? It’s Materials Science, Not Magic.
11:57 minutes
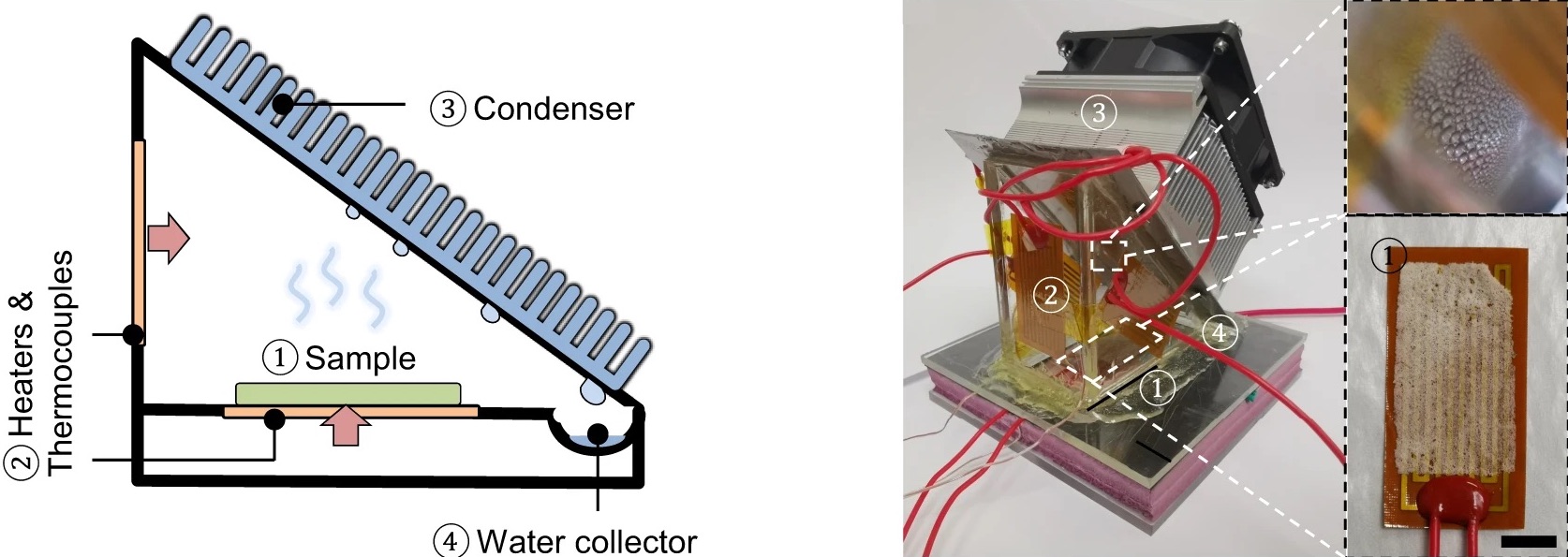
You’ve probably seen a magic trick in which a performer makes a playing card, coin, or even a rabbit appear out of thin air. Writing in the journal Nature Communications, researchers at UT Austin describe an experiment where they seem to pull water out of dry air—but it’s not magic, and it’s not a trick. Carefully applied materials science and engineering allows the team to extract as much as six liters of water per day from one kilogram of their polymer, even in areas with 15% humidity. That’s drier than the Sahara Desert.
The material itself contains two main ingredients. First, a konjac gum, which can be found in Asian cooking, rapidly absorbs water from the air. (In scientific terms, it’s a “hygroscopic material.”) The second ingredient, hydroxypropyl cellulose, responds dramatically to changes in temperature. So at lower temperatures, the team’s polymer film absorbs water, but can rapidly release that water when the film is heated by the sun or artificial heating.
Dr. Guihua Yu, a professor of materials science and mechanical engineering at UT Austin and one of the authors of the report, joins Ira to talk about the material, its applications, and what challenges remain before it can be put into widespread use.
Invest in quality science journalism by making a donation to Science Friday.
Dr. Guihua Yu is a professor of Materials Science and Engineering and Mechanical Engineering in the Texas Materials Institute and UT Energy Institute at the University of Texas at Austin in Austin, Texas.
IRA FLATOW: This is Science Friday. I’m Ira Flatow. You know the old magic trick of pulling a playing card out of thin air? Well, what if you could do that with water, pull it out of thin air efficiently, and even from dry desert air? Given the critical water shortages we’re facing and threatening to get worse in our climate crisis, that would be some trick, wouldn’t it?
Well, a group of engineers at the University of Texas at Austin claims to be able to do that. Joining me now is Dr. Guihua Yu, Professor of Materials Science and Mechanical and Engineering at UT Austin. He works with the Texas Materials Institute and the UT Energy Institute. And his group has developed a material that can literally pull water out of air and as I say, even from desert air. And they describe some of its performance in research published in the journal Nature Communications. Welcome to Science Friday Dr. Yu.
GUIHUA YU: Thank you so much for having me, Ira.
IRA FLATOW: What’s that old phrase, any significantly advanced technology’s equivalent to magic? I mean, is this magic? Tell us about this material?
GUIHUA YU: Oh, sure. So we actually have been working on designing these soft materials. So we call it hydrogel. So hydrogel means– by a scientific term, it’s kind of a polymer, this highly cross-link. So this actually magic material have two chemical ingredients. One of them is doing a function of water harvesting in terms of the hygroscopic property. Hygroscopic means you can absorb water vapor.
So we have this one polymer, [? conjugate ?] [? gum. ?] So you can actually find them actually very commonly in kitchens, especially in Asia. So this [? conjugate ?] [? gum ?] actually can be very efficient in terms of absorbing the water vapor and then store it in their network.
So another ingredient that we designed doing also the other function is for water release. So you can imagine, right? So in our daily life, the desiccants can do the job of dehumidifing our ambient air but really, after water absorption. So it’s very hard for them to release the water back to be useful.
IRA FLATOW: Right.
GUIHUA YU: Yeah. So that– typically, we need to pay large energy penalty, meaning that you need to heat up that desiccant to release the water, so going through this evaporation, condensation. So that’s actually you need to have external electricity to do that. So our magic materials that has the second ingredient, we call a [? Hydroxypropyl ?] Cellulose.
So that HPC is actually very unique in terms of they are responsible to the thermal heat, meaning that if you have a mild temperature change, for example, if you have 80 at night– and then actually, when sun comes in to heat up your gel materials to about 95 Fahrenheit. So that actually temperature change will effectively release the water that you originally stored in the gel. So by that effective absorption, and also effective water release, so we actually found this self-standing gel. They are able to collect the water on their own without any external kind of electricity needed.
IRA FLATOW: So you have this gel that is made out of stuff that’s readily available. You can buy it in the market. And it absorbs water on its own sitting there. And then if you leave it out in the sun getting warm enough, it can heat it up enough, and the water comes pouring out. Would that be correct?
GUIHUA YU: Yes. Yes, exactly. Of course, that’s actually it’s every day you can have only one cycle, right? So we call it passive water harvesting. But you can also do active water harvesting. So you can actually do the multicycles every day.
For example, these materials we design, if you kind of heat it up by some electricity, you can actually even run about 15 to 20 cycles per day. So in fact, we also can increase the water yield per day so that we calculate that at a different relative humidity. So for example, in a desert area, that relative humidity is about 15%. So we can actually release about 6 liters of water per kilogram of our materials, so to be very effectively to be used for really critical water needs.
So our project is funded by Department of Defense. Really the goal is for a soldier to use in extreme kind of conditions. But of course, given this very simple way that we make the materials– and it’s also this very cost-effective materials and the renewable [? cellulose. ?] So we believe that we actually can benefit many society in different areas in the world.
IRA FLATOW: So you can get 6 liters of water from 1 kilogram of your stuff in a desert condition.
GUIHUA YU: Yes.
IRA FLATOW: That’s amazing. You must be surprised yourself how well it works.
GUIHUA YU: Oh, yes, originally we thought that if we have these kind of materials, maybe we can have the equal weight of the water that you can absorb. But like really by designing better, how fast they can release water, so this actually is you have these perfect engineering students. They can design this material to work to their best. So we found that the optimum condition is these actually gel can be cycled multiple times a day. So that’s really kind of boosted the water yield per day.
IRA FLATOW: So you imagine, then, first the military and then maybe all of us buying a device that would contain this material on the store shelf somewhere.
GUIHUA YU: Yes, we think that’s definitely possible because as I mentioned, these materials actually can be made very easily. And once you have the kind of these precursors to make these materials– so simply by mixing them together, so let them react for about a few minutes, so they will be able to form these gel materials. And they actually they will be able to start to collect the water.
Of course, if we design a prototype device, with this layer of materials, we call it absorbent [INAUDIBLE], so that will have kind of the optimum kind of design in terms of that portability and also kind of a high yield. So that actually can be for a disaster relief use. But I imagine that in really kind of in our garage, so people buying these solutions that you can make these materials and actually engineer them together. Then maybe it’s a fun project over the weekends and with kids to make it.
IRA FLATOW: [LAUGHS] I’m sure, though, there must be some patentable or proprietary method you’re using though, or is it not?
GUIHUA YU: Oh, yes, this is actually– it’s– we have a patent in terms of how you can actually use these materials. That material is actually patented. But certainly some of these ingredients in terms of that you can make, it’s really easy. That actually can be made by customers.
IRA FLATOW: Does it have a shelf life? I mean, does it get used up at all? Or is it sort of like– I’m thinking, like, a catalyst, that it doesn’t. Or does it– if you use it too many times, well, we have to go out and buy some more?
GUIHUA YU: Well, that’s a great question, Ira. So we actually tested in the lab conditions over several weeks up to about a month so these gels films that we made and in doing about 15 to 20 cycles per day. So after so many days, several weeks, so they still perform very similarly their fresh state. So we believe these gels, because it’s actually we made it is with these spongy structures.
So you can imagine our kitchen sponge, right? So they are not only just lasting for a few days, but rather at least quite a few weeks, even months. So this actually gels is mechanically also relatively strong. So they are able to hold their strengths for a certain period of time. So if for a longer testings, for a few testings, so we will see how long they can last. But I believe this is actually can be with a sufficient kind of shelf life.
IRA FLATOW: Wow. Now, I know you’re an engineer. And I know as an engineer, you know you don’t get something for nothing, right?
GUIHUA YU: Yes.
IRA FLATOW: So there’s got to be some downside to this or some weakness. What is that?
GUIHUA YU: Yes. Yeah, that’s another great question. So talking about potential challenging, one of the keys is how you can expose these gel films with a very high surface area so to interact with water vapor. So one of the processes we actually made them to host, to keep their hierarchical structure is by freeze drying.
So freeze drying process is actually once the gel form, so we want to get rid of their original solvents. That can be water. It can be either organic solvents. But you want to maintain their hierarchical structure.
So this structural step by freeze drying is actually kind more of a limiting factors in terms of scaling up. So if you just simply by drying them with the hot plate or with other means– so sometimes they structurally may collapse. So the freeze drying step probably will be a determination kind of step how scalable the process is. So we are actually trying to see what the other kind of processes that can be used to maintain the microstructures of these gels to have the highest possible kind of efficiency.
IRA FLATOW: Mm-hmm Are there other applications beyond drinking water? Because you’re obviously you’re making fresh water.
GUIHUA YU: Yes.
IRA FLATOW: You could grow plants with it, right?
GUIHUA YU: Yes, exactly!
IRA FLATOW: What else could you do with it?
GUIHUA YU: So that was actually our earlier idea. We actually, in 2019, we published the first work on this SMAG gel. We call it Super Absorbent Moisture Gels. So that was our first generation of this SMAG gel that can harvest the water from the ambient air.
But we also actually, about two years ago, after our first work, we also turned that SMAG gel into so-called self-watering soil. So simply put is you can turn whatever soil, even dry sand, to be able to sell water. So this, actually– concept is actually a demonstration that can potentially useful for sustainable agriculture. So this self-watering soil, by incorporation chemically modified with our SMAG gel, this water harvesting gel, they are able to irrigate themselves and without any additional water. So really, that’s our kind of a demoing that these gel films not only just for harvesting fresh water, but they can also be beneficial for agriculture.
IRA FLATOW: OK. So when are we going to see this? Self-watering soil is just– my mind is blowing here. When are we going to see this type of material on the market or available?
GUIHUA YU: Oh, yes, you’re right. Ira, so we actually are working with some of the industry partners for more of a few tests because in a university lab, everything that is actually is in a lab scale. So usually we work with limited amount of materials. And we test it in more ideal lab kind of conditions.
But when you’re actually going out for a few tests, and then it’s kind of really open to different areas. So that’s actually– it’s how we’re actually designing the additional experiments to work for a few tests. We really hope is in the next few years, once we have more of these field tests. So we will have the idea what challenges remain for pushing them to be useful and actually that the customer can buy in a warehouse or actually kind of also for farmers, they can use on their own.
IRA FLATOW: This is incredible Thank you Dr. Yu for taking time to talk with us and keep us in your loop about what’s happening, OK?
GUIHUA YU: Sure. Yeah. Thank you for having me and look forward to more interaction and hopefully society will see some of these products in the near future.
IRA FLATOW: Yeah. It looks like you’ve invented something really cool and useful. Dr. Guihua Yu, a Professor of Materials Science and Mechanical Engineering at the University of Texas in Austin.
GUIHUA YU: Thank you, Ira.
Copyright © 2022 Science Friday Initiative. All rights reserved. Science Friday transcripts are produced on a tight deadline by 3Play Media. Fidelity to the original aired/published audio or video file might vary, and text might be updated or amended in the future. For the authoritative record of Science Friday’s programming, please visit the original aired/published recording. For terms of use and more information, visit our policies pages at http://www.sciencefriday.com/about/policies/.
As Science Friday’s director and senior producer, Charles Bergquist channels the chaos of a live production studio into something sounding like a radio program. Favorite topics include planetary sciences, chemistry, materials, and shiny things with blinking lights.
Ira Flatow is the founder and host of Science Friday. His green thumb has revived many an office plant at death’s door.