Grade Level
9 -10
minutes
1- 2 hrs
subject
Physical Science
Activity Type:
visualize radiation, advanced, experiment
All around you, and on every surface of the earth, there is radiation pummeling the atoms that make up the matter that we can see and feel. Even as you read this sentence, you are being bombarded by radiation. Pew! Pew!
But fear not, it’s completely normal. This background radiation is safe. And though it cannot be seen directly, you can build a cloud chamber to help you indirectly observe radiation and begin to understand it.
Target Grades: 9-12+
Content Areas: Physics, Engineering and Technology
Activity Type: Detect radiation, design radiation shielding
Time required: About 1 hour
Next Generation Science Standards: HS-PS1-8
Radiation is any type of wave or particle that transmits energy and includes things like ultraviolet light, alpha radiation (particles made up of two neutrons and two protons), sound (acoustic waves), and x-rays (electromagnetic waves). Radiation that transmits large amounts of energy, called high-energy radiation or ionizing radiation, can change or damage other materials and living cells that it comes into contact with. When describing radiation exposure from man-made sources of radiation like X-rays or nuclear energy plants, what is being described is ionizing radiation.

In small amounts, however, ionizing radiation does not do significant harm, and in fact, it’s a part of our everyday life. Ionizing radiation that naturally occurs on our planet is called background radiation and is a natural part of our planet’s environment. Background radiation can come from all kinds of things, including cosmic rays from outside our galaxy, radioactive materials in the earth like uranium and radon, and even radioactive materials in our body, such as certain kinds of potassium and carbon atoms.
Even though we can’t see these different kinds of radiation directly, we can indirectly observe them when they interact with substances that we can see. Interactions are often physical collisions between a radioactive particle or wave and a non-radioactive atom. Detecting radiation by looking for its interactions with atoms is similar to how you might indirectly observe wind: you can’t see wind itself, but you can see leaves, trees, or plastic bags move in the wind and deduce that the wind is there.
What you'll need to build your cloud chamber
With the right tools, you can observe the interaction of radiation with other substances. Cloud chambers are great tools for indirectly viewing certain types of radiation like the kinds we get from our sun. Here’s how to make one.
The basic gist of a cloud chamber is this:

What you’ll need to build your chamber:
- An adult who can safely use isopropyl alcohol and dry ice
- A crystal clear plastic or glass container with a wide, tight-fitting lid to be your cloud chamber. You’ll want something at least as big as a peanut butter jar or a deli container. If you don’t have a lid, a cookie sheet larger than the mouth of the container will work.
- A durable, absorbent material that you can squish into the bottom of the container. Try felt, wool, or a sponge
- Bubble gum or modeling clay (optional)
- Black paper cut to fit inside the lid of your container
- Dry ice and insulating gloves for safe handling
(find a dry ice distributor here) - A plastic or foam container with a rim that can hold both the dry ice and your cloud chamber. It should be about four times the size of your cloud chamber
- A bottle of 90+% isopropyl alcohol (available at most pharmacies)
- A room, closet, or large box that you can make completely dark for conducting your cloud chamber observations in
- A very bright flashlight (LED flashlight works best)
- A small bowl of warm water that can sit on top of your clear plastic or glass container
- A clock, timer, or stopwatch
- Safety glasses and lab apron
- Optional: a digital camera or cell phone camera
Materials safety notes:
- Isopropyl alcohol is toxic to ingest and also highly flammable. Make sure there are no hot surfaces or open flames in your work area, clean spills promptly, and avoid contact with skin or clothing.
- Direct skin contact with dry ice can cause burns. Avoid direct contact with dry ice by using dry, insulating gloves when you handle it. Since dry ice will produce large amounts of gas on contact with liquids, never store in a glass container and always wear safety goggles and a lab apron.
- Because isopropyl alcohol is flammable, it is important to safely allow any of the remaining alcohol in the cloud chamber to evaporate in a well-ventilated area away from open flame at the end of your experiment.
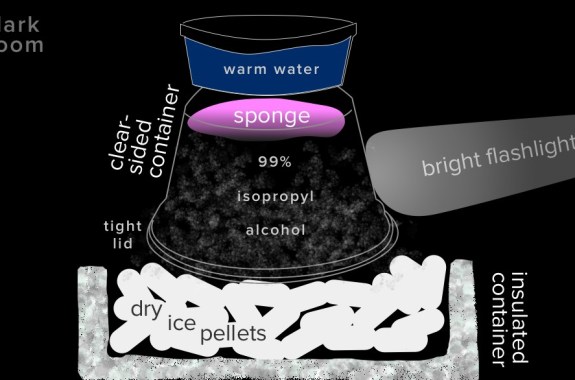
How to build your cloud chamber
- Stuff the bottom of your clear container with your absorbent material. If you need help getting it to stick, try using a small piece of modeling clay or chewed gum to stick it to the bottom. Note: isopropyl alcohol dissolves most adhesives, so you may have to troubleshoot other ways of sticking the absorbent material to the top.
- Set the piece of black paper on the inside of the tightly fitting lid and trim it so that the container can still close with the paper inside.
- Pour just enough isopropyl alcohol into your container so that the absorbent material becomes saturated but is not standing in any liquid. Carefully pour any excess isopropyl alcohol down the sink.
- Turn the container upside-down onto the lid and black paper and secure the lid, making sure that your alcohol-soaked material stays stuck to the bottom of your container. If you don’t have a tight-fitting lid, you can turn your container upside-down onto a cookie sheet, covered with black paper, and tape it around the edges to the cookie sheet. The vapor of the isopropyl alcohol will begin to fill your jar immediately.
- In a room or large box that you can make completely dark, set up your rimmed container on a solid work surface. Using insulated gloves to protect your skin, pour some dry ice into the rimmed container. Place your cloud chamber lid-side down onto the dry ice and keep it there until the lid appears frosty, about 10 minutes. (If you used a cookie sheet, rest the bottom of the sheet on the dry ice.)
- Fill a small bowl or dish with warm water, and set it on top of your cloud chamber. This warms the isopropyl alcohol so the chamber fills with vapor more quickly. You now have a totally cool cloud chamber.
- Turn off all the lights, and shine your flashlight across the bottom of your container through the side. Look inside, what do you see?

Identify the kinds of radiation in your cloud chamber, then measure the amount of background radiation.
See all those little streaks and lines forming in the mist in your cloud chamber? Those are paths from ionizing radiation! Ionizing radiation is high-energy radiation that has enough energy to knock the electrons off of other atoms it collides with. Ionizing radiation can come from cosmic rays from the sun, from the argon in our air, and from radioactive materials. Take a moment to sketch some of the different path shapes that you see. Each of these different path types (e.g., long and straight, short and straight, long and bent) is caused by a different kind of ionizing radiation. You can identify the different types of radiation with this cloud chamber field guide:
 (cloud chamber particle guide, printable version)
(cloud chamber particle guide, printable version)
When ionizing radiation enters a cloud chamber, it interacts with atoms in the atmosphere — like hydrogen, nitrogen, and oxygen–by violently knocking off their electrons. Those atoms turn into positively charged ions, which are very attractive to the gaseous alcohol molecules in the cloud chamber! Chilling the cloud chamber on dry ice causes those gaseous alcohol molecules to crowd so close together that no matter where in the chamber ionizing radiation strikes, there will be many alcohol molecules ready to stick to the trail of positive ions it produces. The result is visible trails of condensed alcohol mist wherever ionizing radiation comes into contact with atoms in the air.
Use your cloud chamber to measure background ionizing radiation.
Using a clock or timer, try to count how many streaks of ionizing radiation you see in your chamber in a minute. Repeat this count two more times, and calculate an average “ionizing interactions per minute” for your cloud chamber. If it helps, you can print out this cloud chamber observation sheet to record your observations and do calculations.
The trails you are seeing in your chamber are a tiny window into the radiation that is buzzing in, through, and around you all of the time. Pretty cool, right?! Well, not if you’re trying to study dark matter.
If you’re looking for dark matter, background radiation is a major problem
Physicists have evidence that in addition to the known subatomic particles that make up most of the things we can see and touch, there is an entirely separate class of very small, potentially weakly interacting particles that make up the majority of our universe called dark matter. Though it comprises over 90 percent of our galaxy, dark matter is poorly understood.
Dark matter is difficult to study because it’s made of unimaginably small particles that we can’t see, and it interacts with other atoms very rarely. Detecting dark matter interactions that are so minute and rare is made especially difficult because they are grossly overshadowed by the background radiation that is constantly pouring down on our planet from cosmic rays. Our planet’s background radiation makes the search for dark matter like trying to hear a shy, whispering child in a party of shouting adults. Science Friday’s video producer, Luke Groskin, visited with scientists looking for dark matter, who describe this conundrum in the video “4850 below.”
Science Friday Documentary: “4850 Below”
In an effort to quiet the “noise” of background radiation, a long-running dark matter experiment called the LUX dark matter experiment (LUX stands for Large Underground Xenon) was built inside a giant water tank in an old mine a mile below the surface of the earth. The tank of water and mile of rock and dirt shield the experiment from background radiation by effectively putting a lot of other atoms – in the form of lots of dense materials like rock and water – between sources of radiation and the experiment.

Engineer your own cosmic ray shielding, and then test it
Now that you have a cloud chamber that works as a particle detector, and a baseline rate of ionizing radiation (“ionizing radiation interactions per minute”), you can make your own radiation shielding and test it by monitoring whether ionizing interactions are less frequent. What will you build around your cloud chamber to shield it from background radiation from sources like the sun?
Materials to try
– Bags or containers of water
– Metal cookie sheets
– Magnets
– Bricks or rocks
– Safe sources of electric fields (e.g., holiday lights)
Effective cosmic ray shielding should do the following:
- Allow you to observe and count the number of ionizing interactions in your cloud chamber, even when the shielding is installed
- Avoid contact with the chamber itself, the dry ice, and the remainder of your experimental setup
- Lower the number of ionizing interactions per minute in your cloud chamber from your initial measurement
To test out your shielding design, first refresh the alcohol in your chamber, and make sure you have sufficient dry ice to keep it cold. As your cloud chamber cools, assemble your radiation shield around it. Once everything is in place, count how many streaks of ionizing radiation you see in your chamber in one minute, and record the count. Count the number of ionizing radiation interactions in a minute two more times, and calculate an average “ionizing interactions per minute” for your cloud chamber with it’s radiation shielding. Is this average different from the rates you observed before you installed the shielding? If you had unlimited funds and space, how would you improve your design to make your shielding more effective?
Related Links
Check out these other cloud chambers for more inspiration:
- Petri-dish cloud chamber from Thomas Jefferson National Accelerator Facility
- Particle detector from Symmetry Magazine
- Compressed air cloud chamber featured at Physicsworld.com
Next Generation Science Standards:
HS-PS1-8 Develop models to illustrate the changes in the composition of the nucleus of the atom and the energy released during the processes of fission, fusion, and radioactive decay.
Educator's Toolbox
Meet the Writer
About Ariel Zych
@arieloquentAriel Zych was Science Friday’s director of audience. She is a former teacher and scientist who spends her free time making food, watching arthropods, and being outside.