Coming Soon: A Germ-Killing Countertop?
6:50 minutes
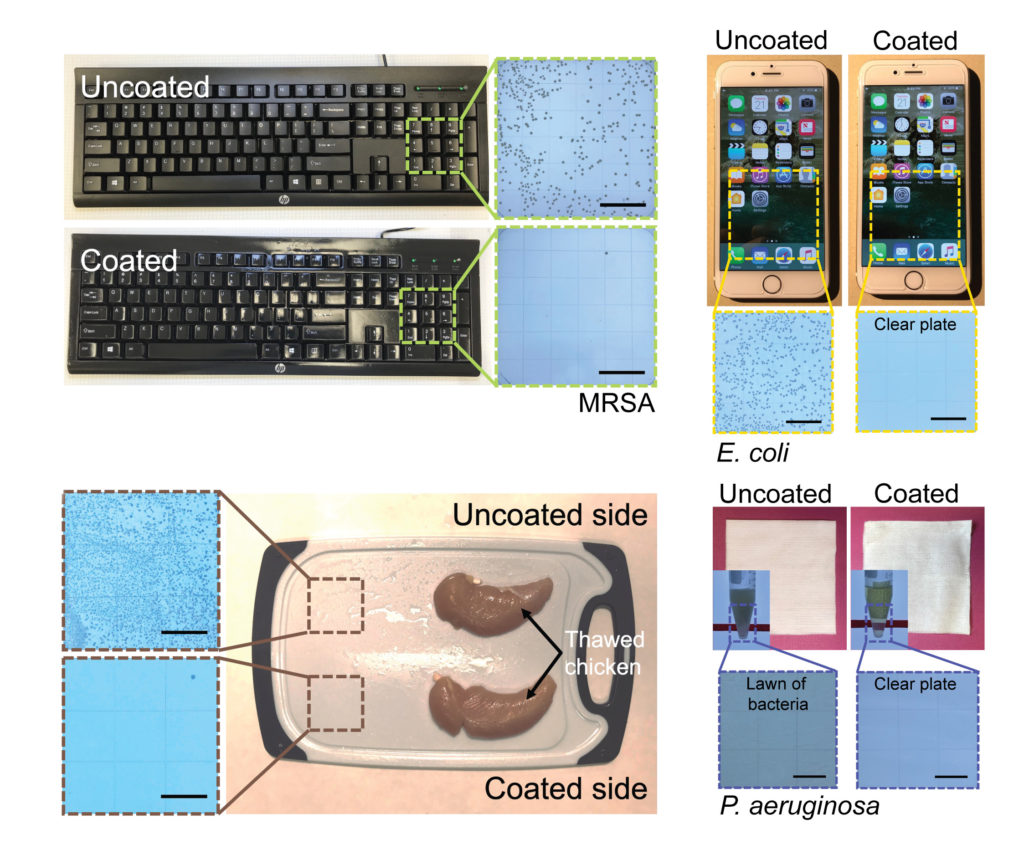
From restaurant tables to office door knobs, not to mention anything inside a hospital, the world is full of surfaces that need sanitizing, lest someone catch a surface-borne viral or bacterial infection like the flu or MRSA. The typical solution involves sanitizing those surfaces with sprays and fluid cleaners. Or, sometimes, using materials that are hostile to microbes, such as silver or copper.
But a team of engineers at the University of Michigan has another solution in mind: a spray-on coating that combines the stabilizing power of polyurethane with the well-documented germicidal qualities of essential oils such as cinnamon, tea tree, and lemon. As the team reports in the journal Matter this week, their coating seems to kill pathogens like SARS-CoV2, MRSA and E. coli within minutes—and lasts for months before it must be refreshed.
Research co-author Anish Tuteja joins Ira to talk about the innovation, and how he thinks it might be useful.
Invest in quality science journalism by making a donation to Science Friday.
Dr. Anish Tuteja is a professor of Materials Science and Engineering at the University of Michigan in Ann Arbor, Michigan.
IRA FLATOW: Remember sanitizing your groceries at the beginning of the COVID-19 pandemic or worrying about picking up the flu from touching a door handle? Oh, yeah. Well, what if your doorknobs and your counters and your cabinets could kill a virus the moment it landed on it? Even deadly MRSA microbes and other communicable diseases.
Germ-killing materials have been an engineering holy grail for decades. But now a team at the University of Michigan has created a coating that seems to work quickly. And it lasts for months without refreshing. And it uses essential oils. Yes.
Here to explain more is my guest, Anish Tuteja. Dr. Tuteja is professor of materials science and engineering at the University of Michigan in Ann Arbor. Welcome to Science Friday.
ANISH TUTEJA: Thank you so much, Ira. It’s a pleasure to join you.
IRA FLATOW: Tell me how you found this coating in the first place.
ANISH TUTEJA: It is certainly a surprising discovery on our end. We were looking at different additives for icephobic coatings or ice-shedding coatings that we work on in my group. And as part of that, we were looking at different essential oils as basically additives that can lower the ice adhesion or improve the ice-shedding capabilities of different polymers. As we were working with these essential oils, we started to realize how effective they were in terms of their antimicrobial capabilities.
And certainly over the last several decades, probably the last 70 years, people have been publishing and reporting on all of the different wonderful antimicrobial properties that different essential oils have. The challenge with all of these has always been the volatility or the evaporation of the essential oils. As all of us know who have interacted with essential oils, these things are very volatile. And they’ll evaporate from a surface in a few minutes. So even though they have excellent antimicrobial properties, they typically don’t last very long on a surface.
We tried to figure out if there was a way of being able to stabilize these essential oils within different polymeric coatings to delay their evaporation rate and essentially make them last for a longer time. The challenge, however, was making sure that you could encapsulate or incorporate them into different polymer films without losing the antimicrobial properties of the essential oils. So the essential oils work by essentially diffusing into different cell membranes for different bacteria and viruses, and we didn’t want to lose the capability of the oils to be able to do that. So that was sort of the major discovery on our end on how do we stabilize these essential oils to be effective and yet persist for a long time.
IRA FLATOW: And you found that you could incorporate them into something we see and use every day– polyurethane, that plastic coating you put on your floors or on your wooden tables.
ANISH TUTEJA: Yeah, that was also one of the things as we started to look at the chemical structure of the essential oils, and it turned out polyurethanes have the right functionality to be able to react partially with some of the essential oils, which allows us to stabilize the remainder of the essential oils. And of course, that provided a whole lot of durability to the coating. These coatings are some of the most durable antimicrobial coatings out there, can survive severe abrasion and wiping and all sorts of different scratching without losing their functionality.
IRA FLATOW: So you just dump the essential oils into polyurethane, mix it together, and spread it on something.
ANISH TUTEJA: Almost that simple, though we make sure that the essential oil actually reacts with the polyurethane. But overall, the process is almost exactly as you described, Ira. We basically mix in three different components into a single solution, spray it or brush it with a paintbrush onto any surface that we want to apply it to, and then just let it sit for a while. And it cures at room temperature, and then it’s ready to go.
IRA FLATOW: Now, we already have ways to kill viruses and bacteria on surfaces. Why is a coating like this so necessary or useful?
ANISH TUTEJA: Yeah, it’s really sort of the antimicrobial surfaces that exist currently fall into these two categories. So if we think about the instant antimicrobials, so anything like a Lysol spray or a wipe, those are essentially instant-kill antimicrobials, so they’ll kill anything, any microbe and virus on the surface in about 1 to 2 minutes. Depending on the microbe, it might be three minutes. But essentially, after you’ve wiped the surface, as we all know, that agent evaporates. And essentially, the surface can be contaminated again. And so you would have to wipe the surface again to be able to decontaminate the surface.
We also have metals, as you were talking about earlier about doorknobs. Copper and brass is very commonly used for doorknobs because it’s really effective in being able to kill microbes. But typically, the time it takes to kill the microbes is really long. So it might take anywhere between 4 to 24 hours before it would kill the microbe.
So let’s say someone sneezes on a surface or has a virus on them, and they breathe or they contact, let’s say, a touchscreen at an airport. The next person coming to that touchscreen at the airport is going to touch it probably in the next few minutes. It’s not going to– there’s not going to be a delay of 4 hours to 24 hours. And so there is that risk of contamination.
So this is where these coatings are so novel in being able to combine both of these properties. They’ll kill anything in a matter of minutes and still persist on the surface for months. And that really is the novel finding in this work.
IRA FLATOW: Does it work on every virus we can think of? Or are there still things we don’t know, you don’t know, that you want to know about it?
ANISH TUTEJA: Yeah, great question. So we’ve only tested about 8 to 10 different bacteria and viruses at this point. We certainly are continuing to work with more. The one sort of positive in all of this regard is all of these oils have evolved over thousands and millions of years to be able to work against a broad spectrum of pathogens. And so we’re essentially sort of taking advantage of what nature has already done. So it does give us some confidence that this is likely to work against a broad spectrum of pathogens.
And we can also mix and match different components, which we have done already, where we can take cinnamaldehyde and combine that with the alpha terpineol, and it seems to work against even a broader spectrum of pathogens than one of these components alone.
IRA FLATOW: Well, Anish, I can’t wait. Thank you for taking time to be with us today.
ANISH TUTEJA: It’s an absolute pleasure. Thank you, Ira.
IRA FLATOW: Dr. Anish Tuteja, professor of materials science and engineering at the University of Michigan. That’s, of course, in Ann Arbor.
Copyright © 2022 Science Friday Initiative. All rights reserved. Science Friday transcripts are produced on a tight deadline by 3Play Media. Fidelity to the original aired/published audio or video file might vary, and text might be updated or amended in the future. For the authoritative record of Science Friday’s programming, please visit the original aired/published recording. For terms of use and more information, visit our policies pages at http://www.sciencefriday.com/about/policies/.
Christie Taylor was a producer for Science Friday. Her days involved diligent research, too many phone calls for an introvert, and asking scientists if they have any audio of that narwhal heartbeat.
Ira Flatow is the founder and host of Science Friday. His green thumb has revived many an office plant at death’s door.