Making Day-Glo Glow More Brightly
9:53 minutes

When you hear the word ‘fluorescent,’ you might think of the colors you see in a psychedelic poster. When you shine a black light on the poster, its colors can seem literally to glow from within. That glow actually comes from chemical fluorescence—the ability of some compounds to absorb energy in one part of the light spectrum, and re-emit it in another part of the spectrum that humans can see. In the case of the black-light poster, ultraviolet radiation from the black light is shifted into the poster’s eye-popping colors.
Over the years, researchers have created thousands of chemical dyes that fluoresce in every color of the rainbow—but there’s a catch. Most of those dyes fluoresce most brightly when they’re in a dilute liquid solution. Now, researchers say they’ve created what they call a “plug-and-play” approach to locking those dyes into a solid form, without dimming their light.
The new strategy uses a colorless, donut-shaped molecule called a cyanostar. When combined with fluorescent dye, cyanostar molecules insulate the dye molecules from each other, and allow them to pack closely together in an orderly checkerboard—resulting in brightly-fluorescing solid materials.
Amar Flood, a professor of chemistry at Indiana University, says the new materials can be around thirty times brighter than other materials on a per-volume basis, and the approach works for any number of off-the-shelf dyes—no tweaking required. Flood joins SciFri’s Charles Bergquist to discuss the work and possible applications for the new technology.
Invest in quality science journalism by making a donation to Science Friday.
Amar Flood is a professor of Chemistry at Indiana University in Bloomington, Indiana.
IRA FLATOW: When you hear the word “fluorescent,” you might think of Day-Glo colors you see in something like a psychedelic rock poster under black light, right? The colors can seem literally to glow from within.
Over the years, researchers have created thousands of chemical dyes that fluoresce in every color of the rainbow. And now a new approach to working with those dyes can produce some of the brightest fluorescent materials yet. Science Friday’s Charles Bergquist has more.
CHARLES BERGQUIST: In the Minerals Gallery of the Smithsonian’s National Museum of Natural History, there’s a case of samples collected from the Sterling Hill Mine in New Jersey. Under regular light, they don’t look like much. They’re gray rocks. But when the black light clicks on, they glow bright neon orange, green, and blue– until the black light clicks off again, and they’re plain, gray rocks.
The minerals in that display are able to fluoresce. They’re absorbing the ultraviolet radiation of the black light and shifting it into a different part of the spectrum that humans can see. Chemists have been able to duplicate some of those effects using chemicals called “dyes.” But it’s harder than you might think. Most of those fluorescent dyes shine best when they’re in a dilute liquid solution. They don’t work as well in solid form. And that can make them hard to work with.
Now researchers say they’ve created a way to lock those dyes into a solid without dimming their light. Joining me now to talk about it is Amar Flood a professor of chemistry at Indiana University in Bloomington and co-author of an article on the research published this week in the journal CHEM. Welcome to Science Friday, Dr. Flood.
AMAR FLOOD: It’s fantastic to be here.
CHARLES BERGQUIST: What’s happening when something fluoresces? Why do some things glow and others don’t?
AMAR FLOOD: Great question. The dyes that we work with, they absorb light. That’s what we readily see. That’s the colored dye stuffs that color clothes. But when a dye can fluoresce, it holds onto that light momentarily as energy, and then re-releases it as light. So molecules that fluoresce have the property of being very efficient at returning the energy it absorbed back as light.
CHARLES BERGQUIST: And just to be clear, this is different from the chemical glow of a firefly or those glow sticks that you play with?
AMAR FLOOD: That’s correct. That’s a chemiluminescence, or a chemical– or in fireflies, a biochemical event is converted into light. For the fluorescent materials that we work with, you have to energize them with UV light. That’s how they pick up that energy and then re-radiate it.
CHARLES BERGQUIST: People talk a lot about fluorescent highlighters or fluorescent safety clothing. How many of those things are actually fluorescent, technically?
AMAR FLOOD: I did a couple of experiments myself with a range of highlighter pens. I put them down on a piece of paper. I looked at them under UV light. And it turns out that half of them are fluorescent and the other half are not. So they all had this property of popping off the page. But maybe half of them are fluorescent.
CHARLES BERGQUIST: Why is it hard to make something solid fluorescent?
AMAR FLOOD: You mentioned the minerals at the beginning. And they are inspiring, because they get us to think, hey, look, if we could uncover the rules that govern the fluorescence in that material, maybe I could make a new material that uses those same rules, but I could be in charge of the color. I could be in charge of the lifetime over which it fluoresces. It turns out we’ve learned the rules, often. But it’s hard to make new materials that enlist those rules.
And there are two pieces of the puzzle here. When you try and make them into solids by growing a crystal, they don’t often behave like we would like them to behave. They don’t form a nice, say, checkerboard arrangement. So that’s a very difficult area of science that could, at any point, thwart your efforts to take or transfer a fluorescent dye into a fluorescent solid.
So that’s getting the packing of the molecules just right. The reason why that matters is because, generally, if you just let the fluorescent molecules, in our case, pack however they want, willy-nilly, they typically come intimately close to each other.
And this is where I sort of liken it to small children in a classroom. They’re great as individuals. But you get them together, then they start fidgeting and interfering with each other. And they stop behaving as a unique person and take on a life of their own. And when you do this with fluorescent molecules, when you put them together, they stop fluorescing.
The other thing that they do is, they change color. You might have your fluorescent compound. And you look at it in the dilute solution you mentioned at the beginning, and they appear red. And then when you make them into a solid, you’re expecting a red solid, and you get a green product out the other end.
So can you control the way the molecules pack together? And if they do pack together, can you stop the molecules from interfering with their intrinsic fluorescent properties? And that’s really the essence of what we have done in the materials that we are sharing with the world at the moment.
CHARLES BERGQUIST: So in essence, it’s a way to insulate the dye molecules from each other as they pack closer together into this orderly checkerboard lattice?
AMAR FLOOD: Absolutely. COVID-19– the word “socially distance” has popped up. And in this particular case, that’s pretty much what’s happening.
CHARLES BERGQUIST: You’re listening to Science Friday from WNYC Studios.
Once it’s in that stable checkerboard lattice, what can you do with it? Can you put it in paints, plastics? What’s the end goal here?
AMAR FLOOD: So we are exploring throw this in goals are. At the base level, they need to be used in potential technologies where brightness really matters. There’s medical diagnostics, where perhaps you might make a faster and more efficient detection of early disease. There are medical lasers where you might want to be able to change the color out of your laser more easily. And in different types of solar energy technologies, where you might like to capture different areas of the solar spectrum.
But related to the solar energy area are also advanced materials that scientists and engineers are looking at that haven’t yet made it to primetime. One in particular is a phenomenon called “upconversion.” And in essence, what you do is, you put it two low energy photons of light that come from the Sun and normally are too low in energy. You can’t really drive an electrical circuit. And so often, they just hit the Earth’s surface, and they’re not used.
So the dream for upconversion is, hey, look, if we can capture two of those low energy photons, double them together, and create a higher energy state inside a material, can we pull that higher energy out to drive an electrical circuit? And the underlying phenomena of that works really well in solution– works really well in dilute solution. But the moment you put those dyes into the solid, they have the problems that we discussed earlier. They stopped working.
So people in that community have been asking us, hey, look, can we take that solution-based phenomenon of upconversion and transfer it into the solid state using our materials?
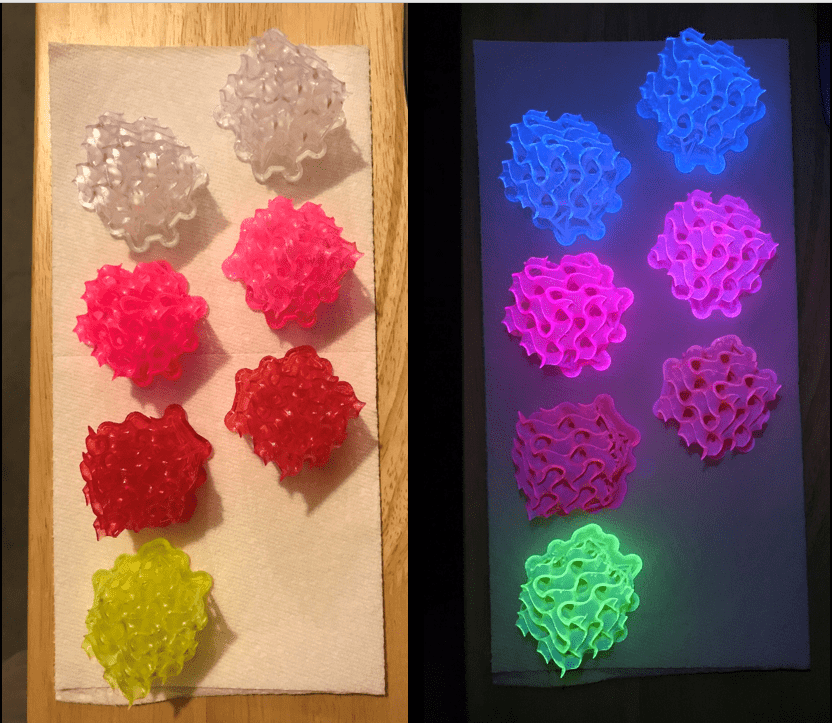
CHARLES BERGQUIST: We have some pictures of some of your sample materials on our website at sciencefriday.com. And they’re these beautifully glowing, twisted structures. How much brighter are your things once you get it into that solid form than a traditional material?
AMAR FLOOD: Those images are of our materials put into polymers. And in terms of brightness, it turns out that when you try and put fluorescent dyes into polymers, it has the same problem. As you increase the amount of the fluorescent dye you put into the polymers, they stop working. They stop fluorescing. And it’s of no use to you.
So what our materials allows us to do is to push right through that limit and to have bright fluorescence where you simply couldn’t have a polymer material fluoresce at all. So that’s one metric in those sort of polymer materials that we’ve got images of.
Another metric comes with those medical diagnostics. The brightnesses are 30 times greater than in existing medical diagnostic particles that go by the name of cadmium selenide.
CHARLES BERGQUIST: Interesting. Well, thank you very much for taking time to talk with me today.
AMAR FLOOD: Thanks. It’s been really nice chatting with you, too, Charles.
CHARLES BERGQUIST: Dr. Amar Flood is a professor of chemistry at Indiana University in Bloomington. For Science Friday, I’m Charles Bergquist.
Copyright © 2020 Science Friday Initiative. All rights reserved. Science Friday transcripts are produced on a tight deadline by 3Play Media. Fidelity to the original aired/published audio or video file might vary, and text might be updated or amended in the future. For the authoritative record of Science Friday’s programming, please visit the original aired/published recording. For terms of use and more information, visit our policies pages at http://www.sciencefriday.com/about/policies/
As Science Friday’s director and senior producer, Charles Bergquist channels the chaos of a live production studio into something sounding like a radio program. Favorite topics include planetary sciences, chemistry, materials, and shiny things with blinking lights.