Flu? There’s A Patch For That
6:42 minutes
What if getting vaccinated for influenza was as easy as slapping on a Band-Aid? Writing this week in The Lancet, researchers report the initial results of a phase one clinical trial of a bandage-like patch that would deliver vaccines by means of about a hundred dissolvable microneedles, which push the vaccine agents past the protective barrier of the skin. Mark Prausnitz, co-author of the study and a professor of chemical and biomolecular engineering at Georgia Tech, says that applying the patch feels something like pressing Velcro against your skin.
[How do you fend off the flu?]
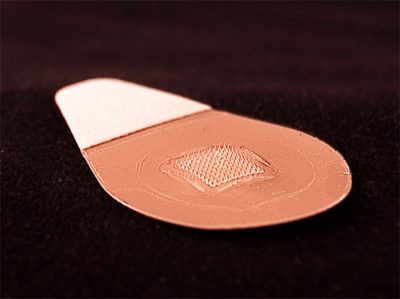
The researchers found that the patches were safe, and produced an immune response similar to that of traditional injections. But unlike regular injected vaccines, the influenza vaccine patch requires no refrigeration, and could also be easily self-administered by a patient at home. Prausnitz cautions that there are still more clinical trials to be done, and also points out that there are regulatory challenges to allowing vaccines to be self-administered outside of a healthcare setting.
[Researchers work towards a universal flu vaccine.]
Mark Prausnitz is a Regent’s Professor in the Department of Chemical & Biomolecular Engineering and the Director of the Center for Drug Design, Development and Delivery at Georgia Tech in Atlanta, Georgia.
IRA FLATOW: This is Science Friday, I’m Ira Flatow. Even though it’s thin and fragile, your skin does a pretty good job at protecting you from unwanted infection. After all, it is your body’s biggest organ, and that protective barrier is why you can’t just rub a vaccine onto your skin like a lotion. It needs to get to the other side of the skin. And normally the way to do that is that big old hypodermic needle.
Well, what if that cross-skin transport could be done more, how shall we say, subtly? Writing this week in the journal The Lancet, a group of researchers at Emory and Georgia Tech describe their initial trials of delivering a flu vaccine via a patch, something like a Band-Aid, studded with about 100 micro needles. Joining me now to talk about that effort and the advantages it could offer is Mark Prausnitz, regents professor in the Department of Chemical and Biomolecular Engineering. Also director of the Center for Drug Design Development and Delivery at Georgia Tech, and he is one of the investigators. Welcome to Science Friday.
MARK PRAUSNITZ: Thank you.
IRA FLATOW: Give me a– give me a radio view of the patch. What does it look like?
MARK PRAUSNITZ: What it looks like to the eye is something, as you said, like a Band-Aid. It’s about an inch across, has a little tab on it for you to grab onto, so you can press it to the skin, push down, and then leave it in place for a few minutes. Now, if you zoomed in microscopically, you would see that there are hundreds conically shaped micro needles that are a few hundred microns tall that have penetrated into the skin and are dissolving in the skin, releasing encapsulated vaccine.
IRA FLATOW: And so what does it feel like when it’s applied?
MARK PRAUSNITZ: You feel a little something, you’re aware of the pressure of your thumb pushing, and some people describe it like feeling maybe there’s some Velcro on the skin, but people don’t describe it as painful.
IRA FLATOW: And so what happens to the needles after they go into your skin?
MARK PRAUSNITZ: Well, a key feature that we’ve designed into it is that the needles are water soluble, so they simply disappear. After you remove the patch, there’s nothing sharp left on it. It can’t be reused, and it doesn’t pose any more danger than a used Band-Aid.
IRA FLATOW: Wow. And could you put this patch– people put it on themselves, or do you have to have a doctor or nurse do it?
MARK PRAUSNITZ: The objective is that people do it themselves. So in the clinical trial that we just recently completed, one group of people received the flu vaccine patch administered by a nurse, but another group of people did it themselves, and they did it just as well as the trained nurse did.
IRA FLATOW: Is the active agent here any different from the regular flu vaccine? Is it the same stuff?
MARK PRAUSNITZ: It is exactly, so it is one of the commercially available flu vaccines. We reformulated it into a micro needle patch, and in fact, one of the other groups in the clinical trial was this the conventional injected flu vaccine.
IRA FLATOW: Now we always worry about transporting drugs, you know. They go through them in the mail, or they– heat destroys them. Is there a problem if, let’s say, if you wanted to mail this out to your patients? Would it survive that delivery?
MARK PRAUSNITZ: Well, that’s another thing that we considered in the design of the patch, was to make it as stable as possible. So we have shown that the patch is stable without refrigeration. We’ve gone out to one year now at 40 degrees Celsius, and the vaccine is still fully potent. So as a result, you don’t need an ice pack, for example, you could just put your patch in a small padded envelope.
IRA FLATOW: So this is– you’re describing a phase one trial, so you haven’t looked at how effective it is. But you must have some initial findings.
MARK PRAUSNITZ: Sure. This study was– as you’re indicating– the study was designed primarily to look at safety as a first time in humans, but we did measure the immune response after giving the vaccine the various different methods, and what we found is that vaccination with this micro needle patch was just as good as vaccination by the conventional injection by a few different measures of the immune response.
IRA FLATOW: And you’ve already created a company working to commercialize this.
MARK PRAUSNITZ: There is a company called Micron Biomedical which is licensing the technology from Georgia Tech, and is in the process of working not only to commercialize a flu vaccine patch, but delivering other vaccines and drugs using this technology.
IRA FLATOW: Now I would imagine that there’s going to be pushback from the health care industry about a vaccine that doesn’t require a doctor’s office visit for which they could charge.
MARK PRAUSNITZ: Well, there is some pushback. The pushback, I’ve heard, hasn’t primarily been a financial one, but it’s been one of just general concern that vaccines are given in a health care setting, and it’s just unusual for a vaccine to be taken out of it. But at the same time, if we think of the various drugs we can buy over-the-counter and how many of them can actually be quite dangerous if used inappropriate and compare that to a flu vaccine, the flu vaccine is just so much safer.
IRA FLATOW: You know– yes, you’re talking about a flu vaccine, and of course, when we talk about vaccines, we think about the giant universe of vaccination, which of course is leading me to the question of could you generalize this to other vaccines? Can you make other kinds that would work like a patch like this?
MARK PRAUSNITZ: Yes. So the flu vaccine is the one that has advanced the furthest, but coming up behind it is work on a polio vaccine patch as well as a measles and rubella vaccine patch. This is work we’re doing in collaboration with the World Health Organization and the Gates Foundation, and the idea here is not so much that people can self-administer it, which we’d like for flu vaccine, but that it could be– it could be given in mass vaccination campaigns by minimally trained personnel, going house to house.
IRA FLATOW: And so how close are you to actually getting one that’s gone through all the testing and is ready to be distributed?
MARK PRAUSNITZ: Well, the one that’s furthest along is the flu vaccine, and that is– that, we hope, is going to be available for patients to use within five years.
IRA FLATOW: That’s pretty soon, and as you say, this would be the ideal thing to be able to distribute around the world.
MARK PRAUSNITZ: Yeah, so we think of it not only in the US context, but there are even more stringent constraints, let’s say, in developing countries, which while the opportunity for say money to pay for advances is less, the constraints in many ways are even harder.
IRA FLATOW: I’ll bet. Thank you, Doctor Prausnitz for taking time to be with us today, and good luck to you.
MARK PRAUSNITZ: Thank you.
IRA FLATOW: Mark Prausnitz is a researcher at Georgia Tech.
Copyright © 2017 Science Friday Initiative. All rights reserved. Science Friday transcripts are produced on a tight deadline by 3Play Media. Fidelity to the original aired/published audio or video file might vary, and text might be updated or amended in the future. For the authoritative record of ScienceFriday’s programming, please visit the original aired/published recording. For terms of use and more information, visit our policies pages at http://www.sciencefriday.com/about/policies/
As Science Friday’s director and senior producer, Charles Bergquist channels the chaos of a live production studio into something sounding like a radio program. Favorite topics include planetary sciences, chemistry, materials, and shiny things with blinking lights.