The guests at the WorMotel check in with a plop. Each guest has a private room, cozy and controlled temperatures, and enough food to last a lifetime. There’s just one catch: They can never leave.
The WorMotel, a silicone plate with an array of 240 wells that host the roundworm C. elegans, is the creation of researchers at University of Pennsylvania’s Fang-Yen Laboratory. They create the silicone rubber “motels” from a mold, add a splash of bacteria to each “room,” then a machine automatically drops in the guest of honor—a single nematode. Multiple motels are stacked in a carousel, allowing researchers to study thousands of the worms at a time.
“It’s not really been possible to monitor changes in behavior for so many animals automatically,” says Matthew Churgin, a post-doctoral associate at the University of Pennsylvania bioengineering department. “I don’t think it’s possible without the WorMotel.”
[Within sight of the famous New York skyline, you might see something unexpected—whales.]

Churgin worked with Christopher Fang-Yen of the Fang-Yen Laboratory to study the nematodes, and their work was recently published in the journal eLife.
But why study these worms in the first place? And what can an organism that’s just a millimeter long, lives for a matter of weeks, and contains only 1,000 cells tell us about humans?
Nematodes are a popular model organism to study human aging and longevity, explains Churgin. In fact, that’s the ultimate goal of WorMotel.
While they may be tiny, nematodes and humans have some things in common. Like humans, there are 20,000 genes in the worm’s genome, and a number of them have been shown to play similar roles in both species. “And for a lot of those genes, we have no idea what [they] do,” says Churgin. “If you really wanted to see which genes influence something like aging,” he says in a post by Penn Engineering, “you’d want to test them all .”
That’s built into the WorMotel’s design. The bacteria that serves as their food can be engineered to turn off a single, specific gene in each worm, allowing researchers to study how the absence of that gene affects an animal’s lifespan and health as it ages.
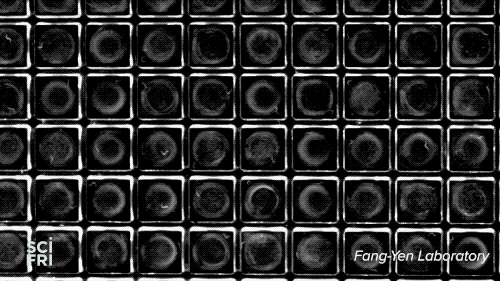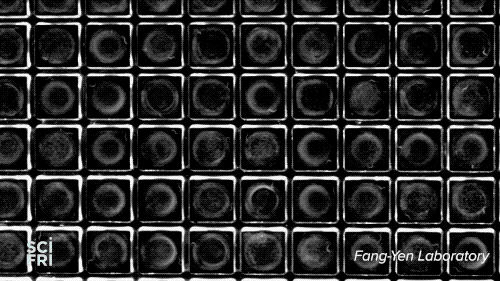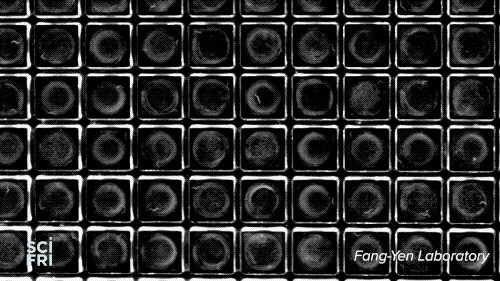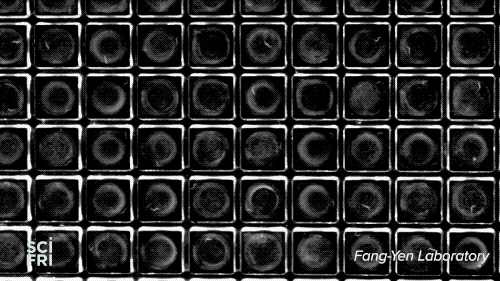
Because researchers want to study each gene, they’ll need to organize the worms into 20,000 groups. “You need about 100 animals per group, so that’s roughly 2 million worms,” explains Churgin—a potentially enormous, long-lived experiment. “But with the WorMotel,” he says, we could do all of those experiments within a few years.”
And hey, life could be worse for the nematodes, too.
“It’s definitely a lot cushier than in the wild,” says Churgin. “In some ways, these worms are pretty privileged, I would say.”
[Researchers in New Zealand are chasing the glow of these worms.]
Credits
Produced by Luke Groskin
Article by Johanna Mayer
Music by Daniel Peterschmidt and Audio Network
Additional Stills Provided by Matt Churgin and Chris Fang-Yen
Special Thanks to the Fang-Yen Laboratory
Related Links
Meet the Producer
About Luke Groskin
@lgroskinLuke Groskin is Science Friday’s video producer. He’s on a mission to make you love spiders and other odd creatures.